Harnessing Electromagnetic-Thermodynamic Material Behaviors for Advanced Biomedical Applications
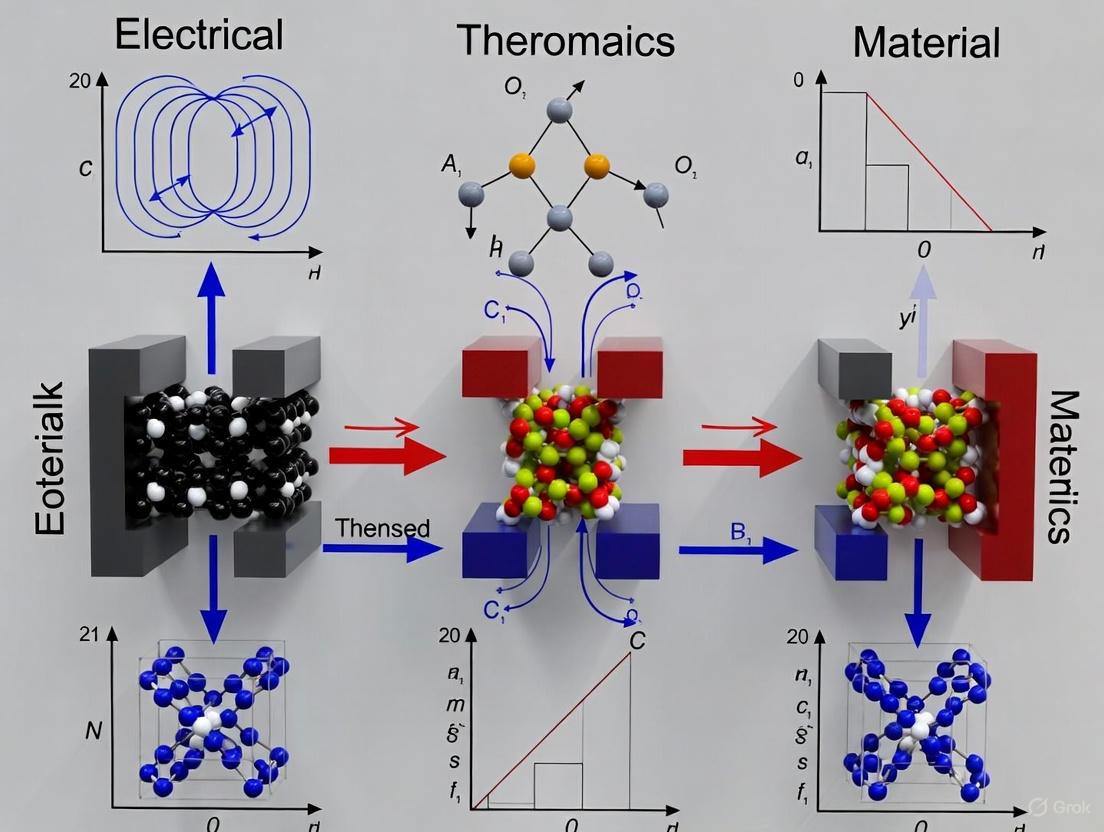
This article synthesizes the latest advancements in understanding and applying the coupled electrical, magnetic, and thermodynamic behaviors of materials for biomedical innovation.
Harnessing Electromagnetic-Thermodynamic Material Behaviors for Advanced Biomedical Applications
Abstract
This article synthesizes the latest advancements in understanding and applying the coupled electrical, magnetic, and thermodynamic behaviors of materials for biomedical innovation. It explores foundational principles of multiferroic, magnetic, and magnetoelectric materials, detailing synthesis and characterization methodologies for applications in targeted drug delivery, tissue engineering, and cancer therapy. The content addresses key challenges in biocompatibility, optimization, and scale-up, while providing comparative analyses of material performance and validation techniques. Aimed at researchers and drug development professionals, this review serves as a strategic guide for leveraging these smart materials to develop next-generation diagnostic and therapeutic platforms.
Fundamental Principles of Coupled Behaviors in Smart Materials
Multiferroic materials, which simultaneously possess two or more ferroic order parameters, and magnetoelectrics, which exhibit coupling between magnetic and electric fields, have emerged as a transformative class of materials in condensed matter physics and materials science. This in-depth technical guide examines the fundamental principles, material systems, characterization methodologies, and underlying thermodynamics governing these advanced materials. Framed within the broader context of electrical, magnetic, and thermodynamic behaviors in materials research, this review synthesizes current understanding of magnetoelectric coupling mechanisms, advanced fabrication techniques for thin films and nanostructures, and characterization protocols. Target toward researchers, scientists, and materials development professionals, this work also explores emerging applications and future research directions that leverage the unique functionalities of magnetoelectric multiferroics, particularly the compelling prospect of electrical control over ferromagnetism at room temperature.
Multiferroic materials are defined by the simultaneous presence of two or more primary ferroic properties—ferroelectricity, ferromagnetism, or ferroelasticity—within a single phase [1]. The magnetoelectric (ME) effect describes the phenomenon where an applied electric field induces a magnetization in the material, or conversely, an applied magnetic field induces an electric polarization [2]. This cross-coupling between magnetic and electrical order parameters enables novel functionalities that cannot be achieved in single-order-parameter materials, with particular interest in low-power electronic devices, advanced sensors, and next-generation memory technology.
The distinction between multiferroic and magnetoelectric materials is fundamental yet often misunderstood. A material can be multiferroic without being magnetoelectric if its ferroic orders do not couple to one another. Conversely, a magnetoelectric material may not be multiferroic if it exhibits coupling between magnetic and electric fields without possessing multiple ferroic order parameters [1]. This nuanced relationship underscores the complexity of these material systems and the importance of precise classification in research and development. The revival of interest in these materials over the past two decades has been driven largely by the technological goal of achieving electrical control of ferromagnetism at room temperature, which would enable transformative advances in spintronics, memory devices, and sensors [3] [2].
Fundamental Principles and Classifications
Order Parameters and Coupling Mechanisms
The fundamental physics of multiferroics and magnetoelectrics centers on the interplay between order parameters and the mechanisms that enable their coupling:
- Ferroelectric Order: Characterized by a spontaneous electric polarization that can be reversed by an applied electric field, typically arising from off-center structural distortions leading to ionic displacement.
- Ferromagnetic Order: Defined by spontaneous magnetization that can be reoriented by applied magnetic fields, resulting from the parallel alignment of electron spins.
- Ferroelastic Order: Manifested by a spontaneous strain that can be reoriented by applied stress.
Magnetoelectric coupling emerges from various microscopic mechanisms, which can be broadly categorized as either intrinsic (single-phase) or extrinsic (composite) [2]. Intrinsic magnetoelectricity occurs in single-phase materials where the coupling is mediated through specific physical mechanisms, while extrinsic magnetoelectricity arises in composite materials where the coupling results from product-property interactions between piezoelectric and magnetostrictive phases.
Table 1: Classification of Magnetoelectric Coupling Mechanisms
| Category | Coupling Mechanism | Representative Materials | Key Characteristics |
|---|---|---|---|
| Intrinsic (Single-Phase) | Spin-driven ferroelectricity | TbMnO₃, TbMn₂O₅ | Magnetic ordering breaks spatial inversion symmetry, inducing polarization |
| Charge ordering | LuFe₂O₄ | Frustrated charge configurations create polar patterns [2] | |
| Geometric ferroelectricity | Hexagonal RMnO₃ (R=Y, Ho-Lu) | Non-centrosymmetric crystal structure enables ferroelectricity independent of magnetism | |
| Hybrid Improper Ferroelectricity | (Ca,Sr)₃Ti₂O₇ | Combination of non-polar rotational modes produces polarization [2] | |
| Extrinsic (Composite) | Product-property | BaTiO₃-CoFe₂O₄ nanocomposites | Strain-mediated coupling between piezoelectric and magnetostrictive phases [2] |
| Exchange bias | Ferromagnetic/Ferroelectric heterostructures | Interface-driven coupling effects |
Thermodynamic Framework
The thermodynamics of multiferroic systems provides a unified framework for understanding multicaloric effects—the thermal response to applied electric and magnetic fields. The general thermodynamic potential for a magnetoelectric multiferroic can be expressed as:
Φ = Φ₀ + αP² + βM² + γ₁P⁴ + γ₂M⁴ + δP²M² - E·P - H·M
where P is the polarization, M is the magnetization, E is the electric field, H is the magnetic field, α and β are harmonic coefficients, γ₁ and γ₂ are anharmonic coefficients, and δ represents the magnetoelectric coupling coefficient [4].
This Landau-type expansion captures the essential physics of coupled order parameters, with the magnetoelectric coupling term (δP²M²) enabling the cross-control of magnetic properties by electric fields and vice versa. The multicaloric effect in such systems comprises contributions from caloric effects associated with each ferroic property plus a cross-contribution arising from their interplay [4]. This framework has been successfully applied to diverse multiferroic classes, including metamagnetic shape-memory alloys and ferrotoroidic materials.
Material Systems and Synthesis Methods
Single-Phase Multiferroics
Single-phase multiferroics represent the fundamental pursuit of intrinsic magnetoelectric coupling, though their realization is challenged by competing electronic requirements for ferroelectricity (typically requiring empty d-orbitals) and ferromagnetism (typically requiring partially filled d-orbitals) [2]. Several material families have emerged as important single-phase systems:
- BiFeO₃: Perhaps the most studied single-phase multiferroic, exhibiting both ferroelectricity (TC ≈ 1100K) and antiferromagnetism (TN ≈ 640K) at room temperature. Its ferroelectricity originates from the stereochemical activity of Bi³⁺ 6s² lone pairs, while magnetism arises from Fe³⁺ cations.
- Hexagonal Manganites (h-RMnO₃, where R=Y, Ho-Lu): These materials display geometric ferroelectricity resulting from tilting of MnO₅ polyhedra and trimerization of rare-earth ions, coupled with antiferromagnetic ordering.
- Spin-induced Multiferroics (TbMnO₃, MnWO₄): In these materials, magnetic spiral structures break spatial inversion symmetry, inducing ferroelectric polarization through inverse Dzyaloshinskii-Moriya interaction.
- Hybrid Improper Ferroelectrics: Materials such as (Ca,Sr)₃Ti₂O₇ achieve ferroelectricity through a combination of two non-polar rotational modes, providing a general design strategy for creating new multiferroics [2].
Composite Magnetoelectrics
Composite magnetoelectrics circumvent the inherent limitations of single-phase materials by combining separate piezoelectric and magnetostrictive phases that interact through strain mediation:
- Horizontal Multilayers: Thin-film heterostructures with alternating piezoelectric (e.g., BaTiO₃, PZT) and magnetostrictive (e.g., CoFe₂O₄, Terfenol-D) layers that couple through interface strain.
- Vertical Nanostructures: Self-assembled nanostructures such as CoFe₂O₄ pillars in BaTiO₃ matrix, where the large surface-to-volume ratio enhances strain transfer [3] [2].
- Polymer-Based Composites: Flexible magnetoelectric composites incorporating piezoelectric polymers (PVDF) with magnetostrictive particles, enabling applications in flexible electronics [5].
- Functionally Graded Composites: Recently developed materials with spatially varying composition to enhance magnetoelectric response across specific temperature ranges [5].
Table 2: Comparison of Major Multiferroic and Magnetoelectric Material Systems
| Material System | Type | Ferroelectric T_C | Magnetic TN/TC | ME Coupling Coefficient | Room Temperature Functionality |
|---|---|---|---|---|---|
| BiFeO₃ | Single-phase | ~1100 K | ~640 K (T_N) | Weak intrinsic | Yes |
| BaTiO₃-CoFe₂O₄ | Composite | ~400 K (BaTiO₃) | ~790 K (CoFe₂O₄) | High extrinsic (~1 V/cm·Oe) | Yes [2] |
| TbMnO₃ | Single-phase | ~28 K | ~41 K (T_N) | Strong intrinsic | No |
| Pb(Zr,Ti)O₃-Terfenol-D | Composite | ~650 K (PZT) | ~380 K (Terfenol-D) | Very high extrinsic (>10 V/cm·Oe) | Yes |
| h-YMnO₃ | Single-phase | ~1270 K | ~70-130 K (T_N) | Weak intrinsic | Partial (FE only at RT) |
Synthesis and Fabrication Protocols
The synthesis of high-quality thin films and nanostructures has been instrumental in advancing multiferroics research [3]. Key fabrication methodologies include:
Pulsed Laser Deposition (PLD) for Complex Oxide Thin Films
Objective: Epitaxial growth of high-quality multiferroic oxide thin films (e.g., BiFeO₃, BaTiO₃) with controlled stoichiometry and crystallographic orientation.
Protocol:
- Target Preparation: Synthesize ceramic target of desired composition through solid-state reaction of precursor oxides with appropriate stoichiometry.
- Substrate Preparation: Select appropriate single-crystal substrates (e.g., SrTiO₃, MgO, LSAT) with suitable lattice matching. Prepare substrate surface through ultrasonic cleaning in organic solvents and thermal annealing in oxygen atmosphere.
- Deposition Parameters: Set substrate temperature to 600-800°C; maintain oxygen pressure of 100-300 mTorr; utilize KrF excimer laser (λ=248 nm) with energy density of 1-2 J/cm² and repetition rate of 1-10 Hz.
- Post-deposition Annealing: Anneal films in oxygen atmosphere at 400-600°C for 30-60 minutes to optimize oxygen stoichiometry and reduce point defects.
This approach has enabled the creation of atomically engineered ferroic layers that function as room-temperature magnetoelectric multiferroics [2].
Self-Assembly of Nanocomposite Structures
Objective: Fabricate vertical heterostructures with magnetostrictive nanopillars embedded in piezoelectric matrix.
Protocol:
- Target Design: Prepare composite targets with appropriate phase ratio or utilize sequential deposition through masked approaches.
- Immiscible System Selection: Identify material pairs with limited solid solubility (e.g., CoFe₂O₄-BaTiO₃) to enable phase separation during growth.
- Epitaxial Stabilization: Optimize deposition temperature (typically 650-800°C) to promote simultaneous crystallization of both phases while maintaining vertical alignment.
- Morphology Control: Regulate pillar size and spacing through manipulation of deposition rate (0.1-1 Å/s) and substrate orientation.
Such nanopatterned hybrid materials have demonstrated room-temperature magnetic switching of electric polarization [2].
Characterization Techniques and Experimental Protocols
Structural and Microstructural Characterization
X-ray Diffraction (XRD) and Reciprocal Space Mapping (RSM)
Purpose: Determine crystal structure, phase purity, epitaxial relationships, and strain states in multiferroic thin films and heterostructures.
Experimental Protocol:
- Utilize high-resolution X-ray diffractometer with Cu Kα radiation source (λ=1.5406 Å).
- Perform θ-2θ scans to identify phase composition and out-of-plane lattice parameters.
- Conduct ω-scans (rocking curves) to assess crystalline quality and mosaic spread.
- Perform φ-scans to determine in-plane orientation relationships between film and substrate.
- Collect reciprocal space maps around asymmetric reflections to quantify strain states and relaxation mechanisms.
Expected Outcomes: For high-quality epitaxial BiFeO₃ films on SrTiO₃(001), (00l) reflections should appear without secondary phases, rocking curve FWHM values typically <0.1°, and RSM should demonstrate coherent or partially relaxed strain states.
Ferroelectric and Dielectric Characterization
Polarization-Electric Field (P-E) Hysteresis Measurements
Purpose: Quantify ferroelectric properties including spontaneous polarization, coercive field, and switching characteristics.
Experimental Protocol:
- Electrode Fabrication: Deposit top electrodes (Pt, Au) through sputtering or electron-beam evaporation with shadow masks (100-500 μm diameter).
- Measurement Setup: Utilize standardized ferroelectric test system (e.g., Radiant Technologies, AixACCT) with virtual ground circuit.
- Measurement Parameters: Apply triangular voltage waveforms at frequencies of 100 Hz-10 kHz with amplitudes sufficient to observe saturation polarization.
- Temperature Dependence: Conduct measurements across temperature range (80-700 K) using temperature-controlled stage to assess phase transitions.
Data Interpretation: For BiFeO₃ thin films, typical room-temperature values include remanent polarization (Pr) of 60-100 μC/cm² and coercive field (Ec) of 100-300 kV/cm.
Magnetic and Magnetoelectric Characterization
SQUID Magnetometry
Purpose: Measure magnetic moment as a function of applied field, temperature, and orientation to determine magnetic ordering temperatures, susceptibility, and anisotropy.
Experimental Protocol:
- Sample Mounting: Secure sample using non-magnetic holder (quartz, plastic straw) with specific orientation relative to applied field.
- Zero-Field Cooling/Field Cooling: Collect M(T) data under ZFC and FC conditions (typically 5-1000 Oe) to identify magnetic transitions and irreversibility.
- Hysteresis Loops: Measure M(H) at relevant temperatures with fields up to ±7 T to determine saturation magnetization, coercivity, and remanence.
Magnetoelectric Coupling Coefficient Measurement
Purpose: Directly quantify the magnetoelectric response by measuring induced polarization under applied AC magnetic field or induced magnetization under applied AC electric field.
Experimental Protocol:
- Sample Preparation: Polish and electrode sample appropriately for electrical measurements; ensure uniform magnetic field exposure.
- Setup Configuration: Place sample between Helmholtz coils for AC magnetic field application (frequency typically 1-10 kHz); simultaneously shield from electromagnetic interference.
- Lock-in Detection: Measure induced voltage at the frequency of the applied AC magnetic field using lock-in amplifier referenced to the field frequency.
- DC Bias Superposition: Apply DC magnetic bias field (0-1 T) while measuring AC magnetoelectric response to determine field dependence.
The magnetoelectric coefficient is calculated as αME = δE/δH = Vout/(t·f·HAC), where Vout is the measured voltage, t is sample thickness, f is frequency, and H_AC is the applied AC magnetic field amplitude.
Visualization of Magnetoelectric Coupling and Material Design
The following diagrams illustrate key concepts and relationships in multiferroic and magnetoelectric materials.
Magnetoelectric Coupling Mechanisms
Multiferroic Materials Classification
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Essential Materials and Reagents for Multiferroics Research
| Material/Reagent | Function/Application | Key Characteristics | Representative Examples |
|---|---|---|---|
| Bismuth Ferrite (BiFeO₃) | Single-phase multiferroic model system | Room-temperature multiferroic; Rhombohedral perovskite; G-type antiferromagnet | Epitaxial thin films; Polycrystalline ceramics; Nanostructures |
| Barium Titanate (BaTiO₃) | Piezoelectric matrix in composites | Ferroelectric T_C ≈ 400K; Large piezoelectric coefficient | BaTiO₃-CoFe₂O₄ nanocomposites; Multilayer heterostructures |
| Cobalt Ferrite (CoFe₂O₄) | Magnetostrictive phase in composites | Inverse spinel structure; Large magnetostriction; High coercivity | Embedded nanopillars in BaTiO₃; Core-shell nanoparticles |
| Lead Zirconate Titanate (PZT) | High-performance piezoelectric | Large piezoelectric coefficients; Morphotropic phase boundary composition | PZT-Terfenol-D composites; Thin film heterostructures |
| Terfenol-D (TbₓDy₁₋ₓFe₂) | High-magnetostriction alloy | Giant magnetostriction (λ_s > 1000 ppm) at room temperature | Laminated composites; Polymer-matrix composites |
| Strontium Titanate (SrTiO₃) | Single-crystal substrate | Perovskite structure; Lattice matching for epitaxial growth | (001), (110), (111) orientations; Nb-doped conducting substrates |
| Polyvinylidene Fluoride (PVDF) | Polymer piezoelectric matrix | Flexible; Solution processable; Moderate piezoelectric response | Magnetoelectric polymer composites; Flexible devices |
Applications and Future Perspectives
The unique properties of multiferroic and magnetoelectric materials enable diverse technological applications:
- Low-Power Memory Devices: Magnetoelectric random access memory (MeRAM) utilizes voltage-controlled magnetic switching to overcome scaling limitations of conventional MRAM, potentially reducing writing energy by orders of magnitude [2].
- Magnetic Field Sensors: Magnetoelectric composites can detect small AC and DC magnetic fields with high sensitivity, applicable in biomedical imaging, navigation, and security systems.
- Energy Harvesting: Multiferroic composites can scavenge ambient electromagnetic energy, converting it to usable electrical power through magnetoelectric transduction.
- Spintronic Devices: Electric field control of magnetic domains enables energy-efficient spintronic logic devices and race-track memories.
- Tunable Microwave Electronics: The electric field control of magnetic permeability in multiferroic heterostructures allows for voltage-tunable inductors, filters, and phase shifters.
Future research directions focus on overcoming current limitations, particularly the enhancement of magnetoelectric coupling at room temperature, development of lead-free environmentally friendly alternatives, and improved understanding of interface coupling mechanisms [5] [2]. The integration of multiferroics with semiconductor technology and the exploration of topological structures such as skyrmions and polar vortices represent particularly promising avenues for both fundamental research and technological innovation [2].
This technical guide has comprehensively examined the fundamental principles, material systems, characterization methodologies, and applications of multiferroic and magnetoelectric materials. Situated within the broader context of electrical, magnetic, and thermodynamic behaviors in materials research, these systems offer exceptional opportunities for scientific discovery and technological innovation. The continued advancement of this field requires interdisciplinary collaboration across materials synthesis, theoretical modeling, device engineering, and characterization science. As research progresses toward stronger magnetoelectric coupling at practical temperatures and the development of scalable fabrication processes, multiferroics are poised to enable transformative technologies that leverage the exquisite control of magnetic properties by electric fields and vice versa, potentially revolutionizing information storage, sensing, and energy conversion technologies.
Magnetic nanomaterials represent a cornerstone of modern materials science, distinguished from their bulk counterparts by their unique electrical, magnetic, and thermodynamic behaviors at the nanoscale. These materials, typically ranging from 1 to 100 nanometers, exhibit novel properties such as enhanced magnetism, superparamagnetism, and high surface-area-to-volume ratios, which are governed by quantum mechanical effects and finite-size phenomena [6] [7]. Their ability to respond to external magnetic fields enables precise control for targeted applications, bridging fundamental research with technological innovation across disciplines from biomedicine to energy and electronics. The classification of these nanomaterials is essential for understanding their structure-property relationships and tailoring them for specific functions, particularly in the context of their electrical, magnetic, and thermodynamic interactions.
This review provides a systematic classification of magnetic nanomaterials, categorizing them into pure metals, metal oxides, and multicomponent systems. It examines their inherent magnetic characteristics, synthesis methodologies, and functional behaviors, framed within a broader thesis on the interplay between electrical, magnetic, and thermodynamic properties in advanced materials research. Special emphasis is placed on their emerging applications in drug delivery, hyperthermia therapy, and diagnostic imaging, highlighting their transformative potential for researchers and drug development professionals.
Classification and Properties of Magnetic Nanomaterials
Magnetic nanomaterials are broadly classified based on their chemical composition and structural configuration. The primary categories include magnetic pure metals, magnetic metal oxides (including ferrites), and multicomponent magnetic nanoparticles such as core/shell structures or nanoclusters [6]. Each category possesses distinct magnetic behaviors, thermodynamic stability, and electrical transport properties, making them suitable for specific applications.
Table 1: Classification and Key Properties of Magnetic Nanomaterials
| Category | Examples | Key Magnetic Properties | Electrical & Thermodynamic Behaviors | Primary Applications |
|---|---|---|---|---|
| Pure Metals | Fe, Co, Ni | Ferromagnetic, high saturation magnetization, large magnetic moment [6]. | High electrical conductivity; susceptible to oxidation, leading to thermodynamic instability; requires protective coatings [6]. | Magnetic separation, data storage [6]. |
| Metal Oxides | Magnetite (Fe₃O₄), Maghemite (γ-Fe₂O₃) | Ferrimagnetic, often superparamagnetic at nanoscale [6] [7]. | Semi-conducting or insulating; inherent thermodynamic stability and biocompatibility [6] [7]. | Biomedicine (MRI, drug delivery, hyperthermia), biosensing [6] [7]. |
| Ferrites | MeFe₂O₄ (Me = Mn, Co, Zn) | Tunable magnetic anisotropy and coercivity based on the metal cation 'Me' [6]. | Variable electrical resistivity; thermodynamic properties can be engineered via composition [6]. | Hyperthermia agents, magnetic cores in electronics [6]. |
| Multicomponent/ Core-Shell | FePt@Fe₃O₄, Co@SiO₂ | Combined magnetic properties (e.g., high magnetization core with protective shell) [6]. | Shell material (e.g., silica, gold) modulates electrical interface and enhances thermodynamic (colloidal) stability in biological environments [6] [7]. | Theranostics, targeted drug delivery, catalysis [6] [7]. |
Magnetic Pure Metals
Nanoparticles of pure magnetic metals such as iron (Fe), cobalt (Co), and nickel (Ni) are characterized by their high saturation magnetization and strong magnetic moments, which are advantageous for applications requiring intense magnetic responses [6]. From a thermodynamic perspective, these metallic systems are highly susceptible to oxidation in air, which can degrade their magnetic performance and limit their utility. This instability necessitates the development of homogeneous, uniform coatings to protect the metallic core from its environment [6]. For instance, iron nanoparticles can be synthesized via the reduction of iron salts in aqueous solutions using sodium borohydride, but the process requires rigorous control over surface passivation to prevent combustion [6].
Magnetic Metal Oxides
Iron oxide nanoparticles, particularly magnetite (Fe₃O₄) and maghemite (γ-Fe₂O₃), are among the most extensively studied magnetic metal oxides due to their favorable biocompatibility, thermodynamic stability, and robust magnetic properties [6] [7]. These materials often exhibit superparamagnetism—a phenomenon where nanoparticles do not retain magnetization in the absence of an external magnetic field, thus avoiding aggregation and enabling their use in biomedical applications like in vivo drug delivery [7]. Their electrical behavior ranges from semiconducting to insulating, which minimizes eddy current losses in alternating fields, a critical factor for hyperthermia therapy. Synthesis methods such as co-precipitation are simple and cost-effective, though they may result in polydisperse particles requiring post-synthesis modifications for improved stability [6] [7].
Multicomponent Magnetic Nanoparticles
Multicomponent nanoparticles, including core/shell structures and magnetic nanoclusters, are engineered to combine the advantageous properties of different materials while mitigating their individual limitations [6]. A common configuration involves a magnetic pure metal or metal oxide core encapsulated within a biocompatible shell, such as silica, gold, or polymers like polyethylene glycol (PEG) [7]. This architecture enhances the thermodynamic (colloidal) stability of the nanoparticles in physiological environments, reduces immune recognition, and provides a versatile surface for functionalization with drugs, targeting ligands, or diagnostic agents [6] [7]. The core dictates the magnetic response, while the shell governs the electrical interface and biological interactions, making these systems particularly powerful for theranostic applications that integrate therapy and diagnosis [7].
Synthesis Methods and Experimental Protocols
The synthesis of magnetic nanomaterials is critical for controlling their size, shape, crystallinity, and ultimately, their magnetic, electrical, and thermodynamic properties. The methods can be broadly categorized into chemical, physical, and biological approaches [7].
Chemical Synthesis Methods
Chemical methods are the most prevalent for producing monodisperse, high-purity nanoparticles with tailored surface characteristics.
- Co-precipitation: This is a simple and cost-effective method involving the simultaneous precipitation of ferrous (Fe²⁺) and ferric (Fe³⁺) ions from an aqueous salt solution in an alkaline medium to form iron oxide nanoparticles (Fe₃O₄ or γ-Fe₂O₃) [7]. The protocol requires strict control over pH, ionic strength, and temperature to achieve desired size and magnetic properties. A typical protocol involves dissolving FeCl₃·6H₂O and FeCl₂·4H₂O in deoxygenated water at a molar ratio of 2:1, followed by the dropwise addition of a base like NH₄OH under inert atmosphere and vigorous stirring. The resulting black precipitate is then isolated by magnetic separation and washed thoroughly [7].
- Thermal Decomposition: This method produces highly crystalline, monodisperse nanoparticles by decomposing organometallic precursors (e.g., iron pentacarbonyl, Fe(CO)₅) in high-boiling organic solvents containing stabilizing surfactants (e.g., oleic acid, oleylamine) [6] [7]. A standard protocol involves heating the precursor solution to 250-300°C under a nitrogen atmosphere for several hours. This allows for precise control over size and shape, but the resulting hydrophobic nanoparticles often require a subsequent ligand exchange phase for biocompatibility [7].
- Sol-Gel Method: This process involves the hydrolysis and condensation of metal precursors (e.g., metal alkoxides) to form an inorganic network, often used for coating magnetic cores with silica or other oxides [7]. For instance, to create a silica shell (Fe₃O₄@SiO₂), magnetic nanoparticles are dispersed in a mixture of ethanol, water, and ammonia, followed by the dropwise addition of tetraethyl orthosilicate (TEOS). The reaction proceeds for several hours, resulting in a core/shell structure that improves stability and provides a surface for further chemistry [7].
Physical and Biological Synthesis Methods
- Physical Methods: Techniques like laser ablation utilize a high-energy laser beam to vaporize a metal target submerged in a liquid medium, producing pure, contaminant-free nanoparticles [7]. Ball milling is a mechanical, top-down approach that grinds bulk magnetic materials into nanosized powders, though it often results in broad size distributions and irregular shapes [7].
- Biological Methods: These eco-friendly approaches use microorganisms (e.g., Magneto spirillum species producing magnetosomes) or plant extracts containing bioactive compounds as reducing and stabilizing agents to form biocompatible nanoparticles [7].
Table 2: Key Reagents and Materials for Synthesis and Functionalization
| Reagent/Material | Function/Application | Example Protocol/Note |
|---|---|---|
| Ferric/Ferrous Chlorides (FeCl₃, FeCl₂) | Iron precursors for co-precipitation synthesis of iron oxides [7]. | Used in a 2:1 molar ratio in aqueous solution under inert atmosphere [7]. |
| Oleic Acid & Oleylamine | Surfactants in thermal decomposition to control growth and prevent aggregation [7]. | Dissolved in high-boiling solvents (e.g., octadecene) with organometallic precursors [7]. |
| Tetraethyl Orthosilicate (TEOS) | Precursor for silica coating via the sol-gel process [7]. | Hydrolyzes to form a uniform SiO₂ shell around the magnetic core, enhancing stability [7]. |
| Polyethylene Glycol (PEG) | Biocompatible polymer for surface functionalization to improve stealth and circulation time in vivo [7]. | Conjugated to the nanoparticle surface via covalent bonding to amine or carboxyl groups [7]. |
| Ammonium Hydroxide (NH₄OH) | Base to initiate precipitation in co-precipitation methods [7]. | Rapid addition under vigorous stirring is crucial for uniform nucleation [7]. |
| Dextran | Natural polymer for coating, enhancing colloidal stability and biocompatibility in biomedical applications [7]. | Often added during or immediately after the co-precipitation reaction [7]. |
Figure 1: Experimental workflow for synthesizing and functionalizing magnetic nanomaterials, showing the main chemical, physical, and biological synthesis pathways leading to surface functionalization and final application.
Advanced Characterization and Interplay of Properties
Understanding the electrical, magnetic, and thermodynamic coupling in magnetic nanomaterials is paramount for predicting their performance in practical applications. Advanced characterization techniques are employed to probe these interconnected properties.
Magnetic and Electrical Characterization
The magnetic properties of nanomaterials, including saturation magnetization, coercivity, and remanence, are typically measured using a vibrating sample magnetometer (VSM) or a superconducting quantum interference device (SQUID) magnetometer [6] [7]. For electrical characterization, impedance spectroscopy is used to measure the complex impedance (resistance and reactance) of materials, which is crucial for understanding their behavior in electronic circuits and under AC fields, such as in hyperthermia applications [8]. A key consideration in such measurements is the effect of Joule heating, where an alternating current (AC) passing through a material causes temperature fluctuations due to electrical resistance. This time-varying heating can lead to non-linear electrical responses and apparent inductance, which must be accounted for to avoid misinterpretation of a material's intrinsic magnetic behavior [8].
Thermodynamic and Magneto-Plastic Coupling
At the nanoscale, the thermodynamic stability of a system is significantly influenced by its surface energy and external fields. Furthermore, in ferromagnetic materials, a strong coupling often exists between magnetic states and mechanical deformation, known as magneto-plastic coupling [9]. Energy-based thermodynamic models have been developed to describe this phenomenon, where the magnetic anisotropy induced by mechanical rolling processes affects both the magnetic and elastic response of the material [9] [10]. This coupling is critical for applications in electrical steel sheets used in motors and transformers, where rotational magnetization and magnetostriction impact energy efficiency and core losses [10].
Applications in Biomedicine and Electronics
The unique properties of magnetic nanomaterials have led to their deployment in a wide array of advanced applications, particularly in biomedicine and electronics.
Biomedical Applications
In biomedicine, superparamagnetic iron oxide nanoparticles (SPIONs) are the most widely used due to their biocompatibility and responsiveness to external magnetic fields [7]. Their applications are multifaceted:
- Targeted Drug Delivery: MNPs can be functionalized with therapeutic agents and guided to a specific site, such as a tumor, using an external magnetic field. This enhances drug concentration at the target site while minimizing systemic side effects [6] [7].
- Hyperthermia Therapy: When subjected to an alternating magnetic field, MNPs dissipate heat. This property is exploited to thermally ablate malignant cells by raising the temperature of the tumor environment to 41–46°C [7].
- Magnetic Resonance Imaging (MRI): SPIONs act as excellent contrast agents in MRI, shortening the transverse relaxation time (T2) of surrounding water protons and producing darker images that improve diagnostic accuracy [7].
- Theranostics: MNPs serve as platforms that combine diagnostic capabilities (e.g., MRI contrast) with therapeutic functions (e.g., drug delivery or hyperthermia) in a single system, enabling personalized medicine approaches [7].
Electronic and Spintronic Applications
Beyond biomedicine, magnetic nanomaterials are pivotal in the development of next-generation electronics. Perovskite materials with the ABX₃ structure (e.g., manganites) exhibit remarkable properties like colossal magnetoresistance (CMR) and multiferroicity, where ferroelectric and magnetic orders coexist [11]. These materials are central to spintronics, a field that utilizes the spin of electrons, in addition to their charge, for information processing [11] [12]. Recent breakthroughs have demonstrated that magnetic waves, known as magnons, in antiferromagnetic materials can generate measurable electric signals [12]. This discovery, which bridges magnetism and electricity without the flow of electrical current, paves the way for computer chips that operate at terahertz frequencies with drastically lower power consumption [12].
Figure 2: Logical relationship between the inherent magnetic properties of nanomaterials, the functions they enable, and their resulting high-impact applications in biomedicine and electronics.
The systematic classification of magnetic nanomaterials from pure metals to complex oxides provides a foundational framework for understanding their electrical, magnetic, and thermodynamic behaviors. This review has delineated how composition, structure, and synthesis protocol dictate these properties and, consequently, the functional application of the materials. While significant progress has been made, challenges remain in the large-scale, reproducible synthesis of monodisperse nanoparticles, the precise control over their surface chemistry, and a comprehensive understanding of their long-term fate in biological and environmental systems. Future research directions will likely focus on the development of more sophisticated multicomponent nanostructures, the exploration of novel magnetic phenomena such as altermagnetism [13], and the deepening of our knowledge regarding magneto-thermal and magneto-electric couplings at the nanoscale. Overcoming these hurdles will be essential to fully unlock the potential of magnetic nanomaterials in revolutionizing fields from personalized medicine to energy-efficient computing.
Thermodynamic Phase Transitions in Magnetic Spin Textures and Their Dynamics
The study of thermodynamic phase transitions in magnetic spin textures represents a frontier in condensed matter physics, bridging the electrical, magnetic, and thermodynamic behaviors of advanced materials. Magnetic metamaterials—artificially engineered systems with tailored magnetic properties—enable the exploration of fundamental physics often inaccessible in naturally occurring crystals [14]. These systems display rich collective phenomena including highly frustrated states, topological protections, and complex phase diagrams governed by competing interactions. Understanding the thermodynamic properties of these magnetic textures is paramount for advancing future technologies in spintronics, quantum computing, and energy-efficient electronics [15] [16].
This technical overview examines thermodynamic phase transitions across diverse magnetic systems, focusing on experimental methodologies for detecting and characterizing these transitions, the underlying theoretical frameworks explaining observed behaviors, and potential applications leveraging these phenomena. The convergence of nanofabrication capabilities, advanced measurement techniques, and theoretical modeling has created a fertile ground for exploring and manipulating magnetic phase transitions in artificial spin systems.
Theoretical Foundations of Magnetic Phase Transitions
Magnetic phase transitions occur when a magnetic system undergoes a qualitative change in its spin configuration in response to varying external parameters such as temperature, magnetic field, or pressure. In frustrated magnetic systems, competing interactions prevent simultaneous minimization of all interaction energies, leading to massive degeneracy and often unexpected emergent behaviors.
Frustration and Degeneracy in Artificial Spin Systems
Artificial kagome spin ice exemplifies frustrated magnetism in engineered systems. This two-dimensional system consists of elongated single-domain nanomagnets arranged on a kagome lattice and coupled via dipolar interactions [14]. Each nanomagnet acts as a macroscopic Ising spin, aligned along its long axis. The geometry prevents simultaneous satisfaction of all interactions, creating a highly frustrated system with a vast number of quasi-degenerate states—the spin ice manifold.
Theoretical work predicts that upon cooling, such systems undergo consecutive phase transitions where long-range dipolar interactions progressively lift the degeneracy of the spin ice manifold [14]. The predicted sequence includes: a high-temperature disordered spin ice phase (Ice I) with only local vertex constraints; an intermediate charge-ordered phase (Ice II); and finally a long-range charge- and spin-ordered phase (LRO) at the lowest temperatures.
Topological Transitions and Skyrmion Formation
Beyond conventional magnetic ordering, certain systems host topological transitions characterized by the emergence of protected spin textures. In Fe/Gd multilayers, competition among dipolar interactions, perpendicular magnetic anisotropy, and exchange interactions leads to the formation of topologically non-trivial structures including bubbles and skyrmions [16]. Unlike in many skyrmion-host materials, this stabilization occurs without significant Dzyaloshinskii-Moriya interaction, highlighting an alternative pathway for topological texture formation.
The phase behavior in such systems exhibits path dependence, where the specific sequence of applied fields and temperatures influences the final magnetic state [16]. This hysteresis and metastability reflect the complex energy landscape with multiple local minima separated by activation barriers.
Experimental Systems and Observed Phase Transitions
Artificial Kagome Spin Ice
Table 1: Phase Transitions in Artificial Kagome Spin Ice [14]
| Phase Name | Temperature Range | Magnetic Characteristics | Transition Type |
|---|---|---|---|
| Ice I (Disordered) | Highest temperatures | Highly degenerate spin ice manifold with local vertex rules | Crossover from paramagnetic regime |
| Ice II (Charge-ordered) | Intermediate temperatures | Partial lifting of degeneracy with charge order | Second-order phase transition |
| LRO (Long-range ordered) | Lowest temperatures | Full long-range charge and spin order | Second-order phase transition |
Experimental realization of artificial kagome spin ice employed nanomagnets of length 63 nm, width 26 nm, and thickness 6 nm, fabricated over large areas (nine 5×5 mm² arrays) to ensure thermodynamic behavior [14]. By varying inter-magnet separation (170 nm for strong coupling, 400 nm for weak coupling), researchers tuned interaction strengths and corresponding transition temperatures.
Muon spin relaxation (μSR) measurements revealed critical temperatures through peaks in the longitudinal relaxation rate (λL) at 35 K and 145 K for the strongly interacting sample, indicating two second-order phase transitions within the spin ice manifold [14]. The transverse relaxation rate (λT) showed changing slopes across these transitions, further confirming the different correlated states.
Van der Waals Magnetic Semiconductor CrSBr
Table 2: Magnetic Properties of CrSBr Thin Layers [15]
| Property | Observation | Measurement Technique | Significance |
|---|---|---|---|
| Magnetic transitions | Antiferromagnetic to ferromagnetic transitions at specific temperatures | Tunneling magnetoresistance (TMR) | Reveals complex magnetic structure responsive to temperature and field |
| Layer-dependent behavior | Unique spin-flip processes in 4-layer vs. 5-layer devices | Vertical tunneling device configuration | Enables property tuning via thickness control |
| Energy-degenerate states | States with identical net magnetization but distinct rectification properties | Bias-dependent TMR measurements | Suggests diode-like behavior dependent on spin configuration |
CrSBr, a van der Waals magnetic semiconductor, exhibits A-type antiferromagnetic order with direct bandgap semiconductor characteristics [15]. Magnetotransport measurements in few-layer CrSBr using vertical tunneling devices revealed that tunneling magnetoresistance can discern spin configurations indistinguishable by other techniques like photoluminescence.
Researchers observed energy-degenerate states with identical net magnetization but distinct rectification properties, manifesting as diode-like behavior at positive and negative bias voltages [15]. In 5-layer CrSBr devices, an intriguing positive magnetoresistive state emerged under in-plane magnetic fields along the b-axis. A one-dimensional linear chain model successfully computed the magnetic states, elucidating the spin configurations responsible for observed transport phenomena.
Fe/Gd Multilayers and Topological Textures
Table 3: Spin Textures in Fe/Gd Multilayers [16]
| Magnetic Texture | Field Range (at 300K) | Identifying Signature | Topological Character |
|---|---|---|---|
| Stripe domains | μ₀H ≤ 110 mT | Distinct coherent spin wave mode (mₛₜ) | Trivial |
| Bubble/Skyrmion (BSK) lattice | 110 mT < μ₀H < 250 mT | Unique breathing mode (mբₛₖ) | Mixed trivial and non-trivial |
| Saturated state | μ₀H ≥ 250 mT | Incoherent magnetization recovery | Trivial |
Fe/Gd multilayers host a rich variety of magnetic textures including topologically trivial bubbles and protected skyrmions [16]. The [Fe(0.35 nm)/Gd(0.40 nm)]₁₆₀ multilayer system exhibits three distinct magnetic phases depending on the applied out-of-plane magnetic field at constant temperature: stripe domains, a bubble/skyrmion lattice, and a saturated single-domain state.
Time-resolved Kerr spectroscopy identified each phase through their coherent spin wave modes, with the BSK lattice exhibiting a characteristic "breathing mode" [16]. The stability ranges of these textures strongly depend on both temperature and magnetic field history, demonstrating path-dependent phase behavior.
Methodologies for Probing Magnetic Phase Transitions
Muon Spin Relaxation (μSR)
Muon spin relaxation serves as a sensitive local probe for detecting weak dipolar fields and critical fluctuations associated with magnetic phase transitions [14]. In this technique, spin-polarized muons implanted in a sample precess in local magnetic fields, with depolarization rates revealing field distributions and dynamics.
For artificial kagome spin ice, muons implanted in a gold capping layer few tens of nanometers above the nanomagnets detected stray fields of approximately 10 Gauss [14]. The technique measures two relaxation rates: transverse (λT, sensitive to both static field distributions and fluctuations) and longitudinal (λL, sensitive only to fluctuations). Peaks in λL indicate critical slowing down at phase transitions, providing unequivocal signatures of second-order transitions.
Tunneling Magnetoresistance (TMR)
Tunneling magnetoresistance measurements probe magnetic configurations through their influence on electron tunneling probabilities [15]. In vertical tunneling devices with CrSBr as the barrier material, electrical resistance changes reflect the relative alignment of magnetic moments between layers.
This technique proved particularly sensitive for distinguishing spin configurations in CrSBr that remained indistinguishable to optical techniques like photoluminescence [15]. The bias-dependent rectification behavior further enabled differentiation of energy-degenerate states with identical net magnetization.
Time-Resolved Kerr Spectroscopy
Time-resolved Kerr spectroscopy detects magnetic spin textures through their coherent dynamics following ultrafast optical excitation [16]. The polar Kerr rotation signal reveals both incoherent demagnetization and recovery processes, along with coherent spin wave modes characteristic of specific magnetic textures.
This technique enabled mapping of (H,T) phase diagrams for Fe/Gd multilayers, distinguishing stripe domains, bubble/skyrmion lattices, and saturated states through their unique frequency signatures [16]. The approach benefits from sensitivity to nanoscale spin configurations without requiring direct real-space imaging.
Research Reagent Solutions and Experimental Materials
Table 4: Essential Materials for Magnetic Spin Texture Research
| Material/Reagent | Function/Application | Key Characteristics | Reference |
|---|---|---|---|
| Permalloy nanomagnets | Building blocks for artificial spin ice | Single-domain, thermally active at room temperature | [14] |
| CrSBr crystals | Van der Waals magnetic semiconductor | A-type antiferromagnetism, direct bandgap | [15] |
| Fe/Gd multilayers | Platform for topological spin textures | [Fe(0.35 nm)/Gd(0.40 nm)]₁₆₀ structure | [16] |
| Pt seed layer | Substrate for multilayer growth | 5 nm thickness, promotes oriented growth | [16] |
| Si₃N₄ membranes | Substrates for LTEM measurements | Electron-transparent for direct imaging | [16] |
| Low-energy muons | Probe for local magnetic fields | Spin-polarized, sensitive to weak fields | [14] |
Signaling Pathways and Experimental Workflows
Phase Transition Detection via μSR
Spin Texture Phase Mapping
Applications and Technological Implications
The controlled manipulation of magnetic phase transitions and spin textures enables numerous technological applications, particularly in information storage and processing. Spintronic devices leverage both charge and spin degrees of freedom for improved efficiency and functionality [15]. The non-trivial topology of skyrmions and related textures offers protection against defects, potentially enabling robust memory elements and unconventional computing paradigms [16].
Magnetic phase transitions also show promise for sensing applications, where subtle changes in temperature or magnetic field induce dramatic resistance changes through transitions between magnetic states [15]. The bias-dependent rectification in CrSBr further suggests potential for spin-based diode elements.
In quantum technologies, the precise control over magnetic phases and their transitions provides a platform for exploring quantum coherence and many-body phenomena. The ability to engineer degeneracies and controlled lifting through temperature or field variations offers routes to quantum模拟 of frustrated systems.
Thermodynamic phase transitions in magnetic spin textures represent a vibrant research area connecting fundamental physics with technological innovation. Experimental advances in artificial spin systems, van der Waals magnets, and topological multilayers have revealed rich phase behaviors including sequential ordering transitions, path-dependent phase stability, and topological protection. Sophisticated probing techniques including μSR, TMR, and time-resolved Kerr spectroscopy enable characterization of these transitions across temperature, field, and excitation conditions.
The integration of these magnetic systems into functional devices promises advances in computing, sensing, and energy technologies. Future research will likely focus on achieving room-temperature stability of topological phases, enhancing tunability through material design, and exploiting dynamic control of phase transitions for novel functionality. As fabrication techniques advance to create increasingly complex magnetic metamaterials, and characterization methods improve to probe faster dynamics and smaller length scales, our understanding and utilization of magnetic phase transitions will continue to deepen and expand.
The Interplay of Lattice Dynamics, Electronic Structure, and Magnetic Properties
The electrical, magnetic, and thermodynamic behaviors of materials emerge from the complex interplay between their lattice dynamics, electronic structure, and magnetic properties. Understanding this relationship is fundamental to advancing materials research, particularly in fields as diverse as energy storage, catalysis, and drug development, where precise material control is paramount. Lattice dynamics govern how atoms vibrate and how heat propagates through a material, directly influencing its thermal conductivity and phase stability. These atomic vibrations interact strongly with the material's electronic structure—the arrangement of energy levels and electron orbitals—which in turn dictates electronic conductivity and optical properties. Simultaneously, the magnetic properties, arising from electron spins and their ordering, can be significantly modulated by both the lattice and electronic degrees of freedom. This tripartite coupling determines key functional characteristics, from a material's response to external magnetic fields to its performance in thermoelectric or spintronic applications. This guide provides an in-depth technical examination of these interconnected phenomena, supplemented by detailed experimental protocols and data presentation standards to enable rigorous research and verification.
Core Theoretical Framework
Lattice Dynamics and Phonons
Lattice dynamics describe the collective vibrational modes of atoms in a crystalline solid. These quantized vibrations, known as phonons, are not merely background perturbations but are active participants in determining electronic and magnetic behavior. The phonon dispersion relation, ω(k), which describes the relationship between the frequency (ω) and wavevector (k) of these vibrational modes, is a fundamental property that can be calculated from first principles using Density Functional Theory (DFT). Key theoretical constructs include the dynamical matrix, whose eigenvalues yield the phonon frequencies, and the phonon density of states (DOS), which provides the number of vibrational modes at a specific frequency. Notably, anomalies in phonon dispersion, such as the Kohn anomaly, can signal strong electron-phonon coupling. The strength of this coupling, quantified by the electron-phonon coupling constant, λ, directly influences phenomena such as electrical resistivity in metals and conventional superconductivity. Furthermore, lattice vibrations mediate spin-lattice interactions, whereby phonons can modulate exchange interactions between localized magnetic moments, leading to effects such as magnon-phonon hybridization.
Electronic Structure Fundamentals
The electronic structure of a material encompasses the allowed electron energy levels and their occupancy. DFT has become the cornerstone for ab initio calculation of electronic properties, enabling the prediction of band structures, density of states, and Fermi surfaces. The Kohn-Sham equations provide a practical framework for approximating the many-body Schrödinger equation, with the choice of exchange-correlation functional (e.g., LDA, GGA, or hybrid functionals) critically impacting accuracy. For strongly correlated systems, such as those containing d- or f-electron elements, methods like DFT+U or Dynamical Mean-Field Theory (DMFT) are often necessary to correctly describe electronic behavior. The calculated electronic DOS reveals whether a material is a metal, semiconductor, or insulator. Crucially, the Fermi surface topology dictates electronic transport properties and determines which phonon modes can effectively scatter electrons. The interplay with lattice dynamics enters through the electron-phonon interaction, which can renormalize electron energies, open band gaps at specific wavevectors, and facilitate phase transitions.
Magnetic Properties and Interactions
Magnetic properties in materials originate from the spin and orbital angular momenta of electrons and their complex interactions. The primary magnetic interactions include:
- Exchange interaction: The quantum mechanical effect responsible for the alignment of neighboring spins, giving rise to ferromagnetism, antiferromagnetism, or more complex magnetic orders.
- Spin-orbit coupling (SOC): An interaction between an electron's spin and its motion, which can lock spin and charge directions, leading to anisotropic magnetic properties and topologically non-trivial band structures.
- Magnetic anisotropy: The dependence of magnetic energy on the direction of magnetization, crucial for stabilizing long-range magnetic order.
The Heisenberg Hamiltonian, H = -Σ Jᵢⱼ Sᵢ · Sⱼ, where Jᵢⱼ is the exchange integral and Sᵢ is the spin angular momentum at site i, provides a simplified model for describing many magnetic systems. The interplay with electronic structure is profound, as the exchange interaction J is itself a consequence of the electronic configuration and interatomic distance. Similarly, lattice vibrations can modulate J, creating a pathway for thermal demagnetization and influencing magnetic phase transitions.
Interplay and Coupling Mechanisms
The coupling between lattice, electronic, and magnetic subsystems gives rise to rich phenomenology:
- Magnetostriction: The deformation of a crystal lattice in response to a change in its magnetization, and its inverse, the variation of magnetic properties with applied strain.
- Magneto-caloric effect: The change in temperature of a magnetic material upon the application or removal of a magnetic field, governed by the coupling between magnetic moments and the lattice thermal bath.
- Spin-phonon coupling: A mechanism where phonons modulate exchange interactions, observable as shifts in phonon frequencies below magnetic ordering temperatures.
- Electron-phonon coupling: The interaction between electrons and lattice vibrations that governs conventional superconductivity, electrical resistivity, and charge-density-wave formation.
- Anomalous Hall effect: A Hall voltage arising from the material's intrinsic magnetism and spin-orbit coupling, rather than the external magnetic field alone.
Table 1: Key Coupling Phenomena in Materials
| Coupling Mechanism | Theoretical Description | Experimental Signature | Functional Consequence |
|---|---|---|---|
| Electron-Phonon | Eliashberg function α²F(ω), coupling constant λ | Kink in ARPES dispersion; Raman linewidth | Superconductivity; Resistivity |
| Spin-Phonon | Hamiltonian: H = Σ ∂Jᵢⱼ/∂u Sᵢ·Sⱼ u (u: atomic displacement) | Frequency shift in phonon spectra below T꜀ | Magnetostriction; Multiferroicity |
| Spin-Orbit | Hamiltonian: H = ξ L·S | Magnetic anisotropy; Anomalous Hall effect | Topological insulators; Spintronics |
The visualization below illustrates the fundamental interactions and experimental characterization methods connecting lattice dynamics, electronic structure, and magnetic properties:
Diagram 1: Core Interplay and Characterization
Experimental Methodologies
Sample Synthesis and Characterization
The foundation of reliable research in this field lies in meticulous sample preparation and characterization. Single-crystal samples are often essential for angle-resolved measurements, while high-quality polycrystalline samples suffice for many bulk property investigations. The synthesis method must be explicitly documented with sufficient detail to enable reproduction, including precursor materials, synthesis atmosphere, thermal treatment profiles, and post-synthesis processing [17]. For materials sensitive to oxidation or hydration, handling procedures under inert atmosphere should be specified.
Upon synthesis, comprehensive structural characterization is imperative. X-ray diffraction (XRD) provides fundamental crystal structure information, phase purity, and crystallite size. The Rietveld refinement method should be employed for quantitative phase analysis and lattice parameter determination. For microscopic analysis, scanning/transmission electron microscopy (S/TEM) reveals morphological features, crystal structure, and elemental distribution via energy-dispersive X-ray spectroscopy (EDS). The experimental section should explicitly state instrument models, operating conditions, and data analysis methods [18]. Specific characterization data should be presented in a standardized sequence: yield, melting point (if applicable), elemental analysis, spectral data (UV, IR, NMR), and mass spectrometry data, following established reporting conventions [18].
Probing Lattice Dynamics
Inelastic neutron scattering (INS) is the most direct technique for measuring phonon dispersion relations throughout the Brillouin zone. INS provides momentum-resolved information about phonon energies and lifetimes. Raman and infrared spectroscopy offer complementary approaches for probing zone-center phonons, with each technique sensitive to different symmetry modes. X-ray scattering can also probe phonons, particularly with the high brilliance of synchrotron sources. For thermal properties, specific heat measurements reveal lattice contributions through the Debye model, while thermal conductivity measurements provide information about phonon transport and scattering processes.
Table 2: Experimental Techniques for Lattice Dynamics
| Technique | Information Obtained | Sample Requirements | Key Parameters |
|---|---|---|---|
| Inelastic Neutron Scattering | Full phonon dispersion, Density of States | Large single crystals (~cm³), Deuteration may be needed | Energy resolution, Momentum transfer |
| Raman Spectroscopy | Zone-center optical phonons, Symmetry | Powder, thin film, single crystal | Laser wavelength, Polarization configuration |
| Infrared Spectroscopy | Zone-center IR-active phonons | Powder pellet, thin film | Spectral resolution, Temperature range |
| Specific Heat | Lattice contribution, Debye temperature | Bulk sample, ~mg to g | Temperature range, Measurement technique (PPMS) |
Electronic Structure Characterization
Angle-resolved photoemission spectroscopy (ARPES) directly measures the electronic band structure, Fermi surface, and many-body effects in materials. With spin-resolution (Spin-ARPES), it can additionally probe spin-texture of bands. Scanning tunneling microscopy/spectroscopy (STM/STS) provides real-space imaging of electronic structure with atomic resolution, capable of mapping local density of states and identifying defects. X-ray absorption spectroscopy (XAS) and X-ray emission spectroscopy (XES) probe element-specific unoccupied and occupied electronic states, respectively. For bulk electronic properties, transport measurements (resistivity, Hall effect, thermopower) provide indirect but crucial information about carrier concentration, mobility, and scattering mechanisms. When reporting spectroscopic data, authors should include instrument frequency, solvent, and standard where applicable, following established conventions for data presentation [18].
Magnetic Property Measurements
Superconducting quantum interference device (SQUID) magnetometry remains the standard for DC magnetization measurements, providing information about magnetic ordering temperatures, susceptibility, and hysteresis. For AC susceptibility, specialized options exist within SQUID systems. X-ray magnetic circular dichroism (XMCD) provides element-specific magnetization information and can separately probe spin and orbital moments. Neutron diffraction is the definitive technique for determining magnetic structures, capable of identifying complex antiferromagnetic, ferrimagnetic, or non-collinear spin arrangements. Mössbauer spectroscopy is particularly powerful for studying iron-containing materials, providing hyperfine parameters that reflect local magnetic environment. For all magnetic measurements, it is critical to report the applied field direction relative to crystal axes and the measurement protocol (e.g., zero-field-cooled vs field-cooled).
The workflow below outlines a comprehensive experimental approach to investigating the interplay between these subsystems:
Diagram 2: Comprehensive Experimental Workflow
Data Presentation and Analysis
Structural and Compositional Data
All experimental data must be presented with sufficient detail to enable verification and reproduction. For structural data from diffraction experiments, lattice parameters with estimated uncertainties should be reported. The crystallographic information file (.cif) for new structures must be deposited in appropriate databases (e.g., Cambridge Structural Database for small molecules) [19]. For elemental analysis, both found and calculated values should be presented in the form: "Found: C, 63.1; H, 5.4. C₁₃H₁₃NO₄ requires C, 63.2; H, 5.3%" [18]. When reporting NMR data, the format should include: "δH(100 MHz; CDCl₃; Me₄Si) 2.3 (3 H, s, Me)" specifying instrument frequency, solvent, standard, chemical shift, integration, multiplicity, and assignment [18].
Spectroscopic Data
Spectroscopic data should be presented with clear peak assignments and interpretations. For IR spectroscopy, report as: "νmax/cm⁻¹ 3460 and 3330 (NH), 2200 (conj. CN), 1650 (CO)" including both wavenumber and proposed assignments [18]. UV-Vis data should follow: "λmax(EtOH)/nm 228 (ε/dm³ mol⁻¹ cm⁻¹ 40 900), 262 (19 200)" specifying solvent, wavelength, and extinction coefficients [18]. For mass spectrometry: "m/z 183 (M+, 41%), 168 (38)" indicating mass-to-charge ratio, ion identity, and relative intensity [18].
Physical Property Measurements
Electrical transport data should include resistivity vs. temperature, Hall coefficient, and thermopower where applicable. All measurements should include error estimates or standard deviations from multiple measurements. For magnetic data, report both field-cooled and zero-field-cooled magnetization when relevant, and include hysteresis loops with clear indication of saturation magnetization, coercive field, and remanence. For thermal measurements, specific heat data should be presented as Cₚ vs. T, with possible separation into electronic and lattice contributions.
Table 3: Representative Physical Property Data for Selected Materials
| Material | Crystal Structure | Magnetic Ordering Temperature (K) | Electrical Resistivity at 300K (μΩ·cm) | Debye Temperature (K) | Dominant Coupling Mechanism |
|---|---|---|---|---|---|
| Fe | bcc | T꜀ = 1043 (Ferromagnetic) | 9.7 | 470 | Exchange (direct) |
| MnF₂ | Rutile | T꜀ = 67 (Antiferromagnetic) | ~10¹² (Insulator) | 450 | Superexchange |
| La₂CuO₄ | Layered Perovskite | T꜀ = 320 (Antiferromagnetic) | Anisotropic | 390 | Superexchange |
| EuO | Rocksalt | T꜀ = 69 (Ferromagnetic) | Metal-insulator transition at T꜀ | 200 | Double Exchange |
| Cr | bcc | T꜀ = 311 (Antiferromagnetic) | 12.7 | 630 | Spin-density wave |
Computational Approaches
First-Principles Methods
Density Functional Theory (DFT) serves as the foundation for most first-principles calculations of materials properties. The choice of exchange-correlation functional is critical, with the Perdew-Burke-Ernzerhof (PBE) functional within the generalized gradient approximation (GGA) being widely used for structural properties. For more accurate electronic structure, particularly band gaps, hybrid functionals (HSE06) or GW approximations are often necessary. For strongly correlated systems, DFT+U incorporates an on-site Coulomb repulsion term to better describe localized d or f electrons. The projector augmented-wave (PAW) method and plane-wave basis sets with appropriate energy cutoffs represent standard technical approaches.
Calculating Specific Properties
Phonon spectra can be calculated using the finite displacement method or density functional perturbation theory (DFPT). The former involves creating supercells with atomic displacements and calculating force constants, while DFPT employs a linear response approach. For electron-phonon coupling, the preferred method involves computing the change in Kohn-Sham potential with respect to atomic displacements. Magnetic exchange parameters Jᵢⱼ can be extracted from DFT calculations by comparing the energies of different magnetic configurations or using the magnetic force theorem. For spin-phonon coupling, calculations typically involve computing phonon frequencies in different magnetic states or applying frozen phonon approaches to magnetic supercells.
Software and Implementation
Multiple software packages implement these computational methods, including VASP, Quantum ESPRESSO, WIEN2k, and ABINIT. Computational details must be thoroughly documented, including pseudopotentials, basis set, k-point mesh, energy convergence criteria, and any U values applied. For reproducibility, computational data and input files should be deposited in appropriate repositories, as required by leading journals [19]. When reporting computational results, authors should include convergence tests with respect to key parameters to establish the numerical accuracy of their predictions.
The Scientist's Toolkit
Table 4: Essential Research Reagents and Materials
| Material/Reagent | Function/Purpose | Technical Specifications | Handling Considerations |
|---|---|---|---|
| High-Purity Elements (e.g., Fe, Mn, Eu) | Starting materials for sample synthesis | 99.99% purity or higher, metal basis | Argon glove box for air-sensitive materials |
| Single Crystal Substrates (e.g., MgO, SrTiO₃) | Epitaxial thin film growth | Lattice matching to target material | Surface preparation (annealing, etching) |
| Deuterated Solvents (e.g., CDCl₃, D₂O) | NMR spectroscopy solvent | 99.8 atom % D | Moisture protection, storage conditions |
| Silicon Calibrant | XRD alignment and calibration | NIST-traceable standard | Surface cleanliness, proper mounting |
| Gadolinium Standard | Calibration of SQUID magnetometers | Temperature and field calibration | Avoid introduction of ferromagnetic impurities |
| Iridium Crucibles | Crystal growth of reactive materials | High-temperature stability, chemical inertness | Pre-cleaning at high temperature |
| Cryogenic Liquids (He, N₂) | Low-temperature measurements | Liquid He-4 (4.2K), Liquid N₂ (77K) | Safety protocols for cryogen handling |
Data Deposition and Reproducibility
Adherence to data sharing policies is essential for advancing materials research. All primary data supporting the conclusions of a study must be made available either through deposition in appropriate repositories or as supplementary information [19]. Specific data types have mandated deposition requirements: crystallographic data should be deposited with the Cambridge Structural Database (CCDC) or Inorganic Crystal Structure Database (ICSD), theoretical input/output files in specialized repositories such as the NOMAD repository, and spectroscopic data in domain-specific databases [19]. The data availability statement has become a mandatory component of scientific publications, requiring precise description of how and where data can be accessed [19]. For computational studies, this includes deposition of input parameters and resulting structures. Authors should be prepared to provide original, unprocessed data to editors and reviewers during peer review if requested [18]. When data access is subject to controlled access due to privacy or ethical concerns, the data availability statement should precisely describe the conditions for access, including contact details and the expected timeframe for response to requests [19]. These practices ensure research verifiability and enable the scientific community to build upon published work.
Key Physical Descriptors Governing Proton Conduction and Ionic Diffusion
The pursuit of clean energy technologies, such as fuel cells and solid-state batteries, has placed the fundamental science of ion transport at the forefront of materials research. Understanding and controlling proton conduction and ionic diffusion is critical for developing next-generation energy converters and storage devices. These processes are governed by a complex interplay of atomic-scale interactions, collective dynamics, and material structure. This technical guide synthesizes current knowledge on the key physical descriptors that dictate ionic mobility, framing them within the broader context of the electrical, magnetic, and thermodynamic behaviors of materials. It provides a comprehensive framework for researchers aiming to design advanced materials with tailored transport properties, detailing fundamental mechanisms, quantitative descriptors, experimental probing techniques, and essential research tools.
Fundamental Mechanisms of Proton and Ion Transport
Ionic conduction in condensed phases occurs through two primary mechanistic classes: structural diffusion (involving the concerted motion of charge carriers relative to the host matrix) and vehicular diffusion (involving the physical displacement of charged species).
The Grotthuss Mechanism and Proton Hopping
The Grotthuss mechanism describes proton transport via a structural diffusion process where protons move through a hydrogen-bonded network by successive bond formation and breaking [20]. This mechanism does not require the physical diffusion of the molecular species to which the protons are attached. In proton-conducting perovskites used in solid oxide fuel cells (SOFCs), the Grotthuss mechanism decomposes into two elementary steps [21]:
- Proton Rotation: The reorientation of an O-H bond around a single oxygen ion within its coordination sphere.
- Proton Transfer: The jumping of a proton from an oxygen ion to an adjacent oxygen ion in a neighboring site.
The rate-limiting nature of these steps is governed by hydrogen bond strength. Generally, proton transfer exhibits a higher energy barrier than rotation and is thus often the rate-limiting step; however, in systems with very strong hydrogen bonds, the energy barrier for rotation becomes significant and non-negligible [21]. The formation of transient, strong hydrogen bonds, facilitated by thermal lattice vibrations (phonons), is crucial for enabling efficient proton transfer [21].
The Vehicle Mechanism
In contrast to the Grotthuss mechanism, the vehicle mechanism involves proton or ion transport via the physical diffusion of a charged carrier vehicle, such as a hydrated proton (H₃O⁺) or an ionic phosphate species (e.g., H₂PO₄⁻) [22]. This mechanism dominates when the hydrogen-bonded network is underdeveloped or when stable, diffusive charged species are present in high concentrations. The conductivity via this pathway is directly linked to the viscosity and structural relaxation dynamics of the medium, as it is governed by the same molecular friction that impedes molecular diffusion [20]. In many practical systems, such as diphosphoric acid, the total ionic conductivity results from a combination of both Grotthuss and vehicle contributions, each accounting for a significant portion of the overall conductivity [22].
Correlated Ionic Hopping and Orientational Memory
In solid-state ion conductors, the fundamental step of diffusion is an ion hop—a rare-event, large-amplitude translation between lattice sites. Recent nonlinear optical studies have revealed that these hops are not always memoryless, Markovian steps as assumed in simple random-walk models [23]. Instead, correlated hopping can occur, where the direction of a subsequent hop is influenced by the preceding one. This correlation leads to a persistence of orientational memory, measured as a transient anisotropy in hopping directions following an impulsive trigger. The relaxation of this anisotropy occurs over a finite timescale (picoseconds to nanoseconds), during which the full entropy of transport is not yet realized. This memory effect signifies that the ionic conduction process can be a multi-step, correlated phenomenon rather than a simple Poissonian process, which has critical implications for accurately predicting and modeling transport properties [23].
Quantitative Descriptors and Material Properties
The efficiency of ion conduction is governed by a set of quantifiable physical descriptors that determine the kinetics and thermodynamics of the transport process.
Table 1: Key Quantitative Descriptors Governing Proton and Ion Conduction
| Descriptor | Definition & Physical Meaning | Impact on Conduction | Typical Range/Values |
|---|---|---|---|
| Activation Energy (Eₐ) | Energy barrier for a hopping event (rotation or transfer). | Determines the temperature dependence of conductivity (Arrhenius law). | Proton transfer: ~0.28 eV in BaHfO₃; Rotation: ~0.15 eV [21]. |
| Attempt Frequency (ν₀) | Pre-exponential factor; vibrational frequency of the ion in its potential well. | Governs the intrinsic rate of hopping attempts. | Proton transfer: ~3000 cm⁻¹; Rotation: ~1500 cm⁻¹ [21]. |
| Hydrogen Bond Strength / Length | Measure of the interaction strength between a proton donor and acceptor. | Dictates the rate-limiting step; weaker bonds favor transfer as the bottleneck. | A critical length distinguishes strong/weak bonds [21]. |
| Glass Transition Temp. (T𝗀) | Temperature where a liquid or soft material becomes a glassy solid. | Below T𝗀, vehicle mechanism is frozen; only Grotthuss may operate. | Varies with material; can be tuned by molecular design [20]. |
| Transient Anisotropy Decay Time | Timescale for the loss of orientational memory after a triggering event. | Measures the persistence of correlated hopping; shorter times suggest faster memory loss. | ~10 ps at 300 K to ~3-4 ps at 620 K in K⁺ β-alumina [23]. |
Table 2: Contributions to Total Conductivity in Different Material Systems
| Material System | Total Conductivity | Grotthuss Contribution | Vehicle Contribution | Dominant Rate-Limiting Step |
|---|---|---|---|---|
| Diphosphoric Acid (H₄P₂O₇) at 160°C | ~0.2 S/cm | ~0.1 S/cm (estimated 50%) | ~0.1 S/cm (estimated 50%) | Proton transfer within H-bond network & diffusion of phosphate ions [22]. |
| Protic Ionic Liquid/Imidazole (Low [Imidazole]) | Composition-dependent | More pronounced | Less dominant | Proton transfer, as imidazole acts as a base pulling protons [24]. |
| Protic Ionic Liquid/Imidazole (High [Imidazole]) | Composition-dependent | Less favored (H-bonds too stable) | Dominant | Vehicle mechanism due to stable, chain-like H-bonding [24]. |
| BaHfO₃ Perovskite at 500 K | Governed by hopping rates | Primary mechanism (sole mechanism) | Not applicable | Proton transfer, due to higher barrier (0.28 eV) vs. rotation (0.15 eV) [21]. |
The relationship between key processes and descriptors in proton conduction can be visualized as a logical pathway, as shown in the following diagram.
Experimental Protocols for Probing Transport Mechanisms
Elucidating the dominant conduction mechanism and quantifying the relevant descriptors requires a combination of advanced experimental techniques.
Terahertz-Pumped Kerr Effect (TKE) Spectroscopy
This nonlinear optical method is used to impulsively trigger and temporally resolve anisotropic ionic hopping, providing direct insight into correlated ion dynamics [23].
Detailed Methodology:
- Pump Pulse: A single-cycle terahertz (THz) pulse (center frequency ~0.7 THz) is used to impulsively align ions or trigger hops along a preferential direction defined by the pump electric field vector.
- Probe Pulse: A delayed, weaker optical probe pulse is sent through the sample in a direction perpendicular to the pump.
- Detection: The transient birefringence (Kerr effect) induced in the sample by the pump is measured by changes in the polarization state of the transmitted probe light.
- Signal Interpretation: The emitted signal is proportional to the time derivative of the material's polarization, which arises from ionic velocities and hopping rates. The measured transient birefringence, therefore, reflects the anisotropy in the directions of subsequent ionic hops. The relaxation of this signal reveals the transient anisotropy decay time, which quantifies the loss of orientational memory.
- Temperature Variation: Conducting experiments at varying temperatures allows for the extraction of the activation energy associated with the hopping process from the temperature dependence of the decay time.
High-Pressure Dielectric Spectroscopy
This technique is employed to decouple the vehicle mechanism from proton hopping by isolating the effects of density and packing from thermal energy [20].
Detailed Methodology:
- Sample Preparation: Acidic ionic liquids (ILs) are synthesized, for example, via a two-step process involving the reaction of 1-methylimidazole with a sultone to form a zwitterion, followed by metathesis with the desired acid [20]. Both anhydrous and water-saturated samples are prepared.
- Dielectric Measurement: The complex permittivity (dielectric constant and loss) of the sample is measured over a broad frequency range (typically mHz to MHz).
- High-Pressure Cell: The sample is placed in a high-pressure cell, and dielectric measurements are repeated under isothermal conditions while systematically increasing the hydrostatic pressure.
- Data Analysis: The pressure dependence of the DC conductivity (σ_dc) and the characteristic relaxation times of molecular motions are analyzed. Since pressure increases packing density without changing thermal energy, it primarily impedes the vehicle mechanism (ion diffusion). A conductivity that is relatively resilient to pressure suggests a significant contribution from the Grotthuss proton hopping mechanism, which is less sensitive to bulk density.
First-Principles Simulations and NMR
Computational and nuclear magnetic resonance (NMR) techniques provide atomic-scale insights into structure and dynamics.
- Ab Initio Molecular Dynamics (AIMD): Simulations of model systems (e.g., pure H₄P₂O₇) are performed to track the trajectories of atoms over time, allowing for the direct visualization of proton hopping events and the analysis of the hydrogen-bond network topology [22]. The energy barriers for elementary steps like proton rotation and transfer can be calculated using methods like the Climbing Image Nudged Elastic Band (CI-NEB) within density functional theory (DFT) [21].
- NMR Spectroscopy: Techniques such as pulsed-field gradient NMR measure the self-diffusion coefficients of specific atomic nuclei (e.g., ^1H). Comparing the proton diffusion coefficient from NMR with the macroscopic conductivity from electrochemical impedance spectroscopy can help distinguish between the Grotthuss and vehicle mechanisms [24].
The workflow for integrating these techniques is summarized below.
The Scientist's Toolkit: Essential Research Reagents and Materials
Research in proton-conducting materials relies on a suite of specialized materials, compounds, and characterization tools.
Table 3: Key Research Reagent Solutions in Proton Conduction Studies
| Reagent / Material | Function and Role in Research | Exemplary Use Case |
|---|---|---|
| Phosphoric Acid & Condensates | Model proton-conducting system with high intrinsic conductivity; explores effects of temperature and hydration. | Studying H₄P₂O₇ to understand conductivity at high T and low RH [22]. |
| Imidazole-Based Protic Ionic Liquids | Tunable, low-volatility electrolytes for fuel cells; allows study of H-bond network and composition effects. | [BMIm-SO₃H][pTS] and [BMIm-SO₃H][MeSO₃] for charge-transport studies [20]. |
| Proton-Conducting Perovskites | Solid-state electrolytes for intermediate-temperature fuel cells (PC-SOFCs); study of Grotthuss mechanism in oxides. | BaHfO₃, SrCeO₃ for probing elementary steps of proton diffusion [21]. |
| β-alumina single crystals | Model fast ionic conductors with 2D conduction pathways; used to probe fundamental hopping dynamics. | Na⁺, K⁺, Ag⁺ β-alumina in TKE studies of picosecond hopping [23]. |
| Deuterated Solvents | NMR-active solvents for sample preparation for NMR spectroscopy, enabling detailed molecular-level analysis. | D₂O for ^1H NMR analysis of synthesized ionic liquids and mixtures [20]. |
The rational design of advanced materials for energy applications hinges on a deep and quantitative understanding of the key physical descriptors governing proton conduction and ionic diffusion. This guide has established that hydrogen bond strength, activation energies for elementary steps, and timescales for memory effects are fundamental controls on transport properties. The emergence of sophisticated experimental techniques, such as terahertz-pumped Kerr effect spectroscopy and high-pressure dielectrics, now allows researchers to move beyond macroscopic conductivity measurements and directly probe the correlated and collective dynamics underlying ion transport. By integrating these insights with first-principles computational modeling and a targeted suite of research materials, scientists are now equipped to deconvolute the complex interplay of Grotthuss and vehicle mechanisms, thereby accelerating the development of tailored materials with superior conductivity for the next generation of energy technologies.
Synthesis, Fabrication, and Biomedical Implementation Strategies
The synthesis of magnetic nanoparticles (MNPs) represents a cornerstone of modern materials research, bridging the fields of nanotechnology, magnetism, and thermodynamics. These nanoscale materials, typically ranging from 1 to 100 nanometers, exhibit unique electrical, magnetic, and thermodynamic behaviors that distinguish them fundamentally from their bulk counterparts [25]. The investigation of these properties is not merely an academic exercise but a critical pathway toward technological advancements in diverse areas including targeted drug delivery, magnetic hyperthermia cancer treatment, magnetic resonance imaging (MRI), biosensing, and energy applications [25] [26] [27].
The intrinsic properties of MNPs—such as superparamagnetism, high surface area-to-volume ratio, and quantum effects—are intensely governed by their synthesis route [28]. The control over particle size, size distribution, shape, surface chemistry, and composition achieved during synthesis directly dictates their magnetic susceptibility, saturation magnetization, and thermal stability [29] [30]. Consequently, developing advanced, reproducible synthesis methods is paramount for tailoring MNPs to specific applications, particularly within the demanding field of biomedicine [27].
This technical guide provides an in-depth examination of three principal synthetic pathways—co-precipitation, thermal decomposition, and sol-gel methods. It places special emphasis on how these techniques govern the ensuing electrical, magnetic, and thermodynamic properties of the resulting nanomaterials, providing researchers and drug development professionals with a foundational framework for material design.
Fundamental Magnetic and Thermodynamic Properties at the Nanoscale
At the nanoscale, magnetic materials undergo a significant transformation in their fundamental properties. When the size of a ferromagnetic particle is reduced below a critical diameter (typically around 10-20 nm for magnetite), it becomes a single magnetic domain [28]. This single-domain state can exhibit superparamagnetism, a phenomenon where the particle's magnetization can randomly flip direction under the influence of temperature, resulting in a net zero magnetization in the absence of an external magnetic field [29] [28].
This superparamagnetic behavior is critically important for biomedical applications because it prevents particle agglomeration after the removal of the external magnetic field and allows for precise control in vivo [27]. The thermodynamic properties of these nanoparticles, particularly their thermal stability and magnetization dynamics, are strongly influenced by their finite size and large surface area. The increased surface-to-volume ratio leads to a significant fraction of atoms residing on the surface, resulting in spin disorder and reduced saturation magnetization (Ms) compared to bulk materials [29]. Furthermore, the magnetic anisotropy energy, which holds the magnetization along an easy axis, becomes comparable to the thermal energy, kBT, at room temperature, leading to the superparamagnetic state [30].
Simulation of these thermodynamic properties requires sophisticated numerical approaches, such as Langevin dynamics and time-quantified Monte Carlo methods, to model phenomena like magnetization reversal across timescales from picoseconds to microseconds [30]. Understanding these core principles is essential for selecting and optimizing synthesis methods to achieve desired performance characteristics.
Synthesis Methodologies
Co-precipitation Method
The co-precipitation method is one of the most straightforward and widely employed techniques for synthesizing magnetic iron oxide nanoparticles, particularly magnetite (Fe3O4) and maghemite (γ-Fe2O3) [25] [28]. This aqueous-based process involves the simultaneous precipitation of Fe²⁺ and Fe³⁺ ions in a molar ratio of 1:2 under a basic atmosphere at room temperature or elevated temperatures [29].
Table 1: Key Experimental Parameters and Their Effects in Co-precipitation Synthesis
| Experimental Parameter | Impact on Nanoparticle Properties | Optimal/Reported Conditions |
|---|---|---|
| Type of Base (BOH) | Influences particle size, agglomeration, and mesoporosity [29]. | NaOH, KOH, NH₄OH, (C₂H₅)₄NOH [29] [31]. NaOH yields higher magnetization vs. NH₃ [31]. |
| pH Value | Critically determines the phase, size, and chemical stability of the particles [29] [31]. | Strongly basic conditions (pH 10-12); pH 10 can yield higher magnetization [29] [31]. |
| Ionic Strength | Affects particle size and electrostatic surface charge [26]. | Controlled by salt concentration; lower ionic strength can favor smaller sizes [26]. |
| Reaction Temperature | Influences crystallization kinetics and final particle size [29]. | Room temperature to 80-90 °C [29] [26]. |
| Iron Salt Precursors | Source of Fe²⁺ and Fe³⁺ ions. Common salts include chlorides, sulfates, and nitrates [29]. | FeCl₂·4H₂O and FeCl₃·6H₂O [29] [31]. |
| Stirring Rate | Affects mixing efficiency and nucleation homogeneity [26]. | Typically vigorous and consistent stirring; exact rate varies [26]. |
| Atmosphere | Prevents oxidation of Fe²⁺ to Fe³⁺, which is critical for maintaining magnetite stoichiometry [29] [31]. | Inert atmosphere (N₂ or Ar) is essential [29] [26] [31]. |
The overall reaction for the formation of magnetite is as follows [29]: [ \ce{2Fe^{3+} + Fe^{2+} + 8OH^- -> Fe3O4 + 4H2O} ]
A critical experimental protocol for synthesizing mesoporous magnetite structures is detailed below [29]:
- Solution Preparation: Dissolve 0.02 mol of FeCl₃·6H₂O and 0.01 mol of FeCl₂·4H₂O in deionized water (e.g., 250 mL) under an inert nitrogen atmosphere. Using degassed solutions is recommended to exclude oxygen.
- Precipitation: Add the alkaline precipitating agent (e.g., NaOH, KOH) to the iron solution. The addition can be performed slowly (e.g., 1.0 mL/min using a precision pump) or rapidly via fast injection. The final pH should be monitored and adjusted to the desired value (e.g., between 10 and 12).
- Reaction and Aging: Maintain the reaction mixture at room temperature or heat to 70-80 °C for 20-60 minutes with vigorous stirring under a continuous N₂ flow.
- Product Isolation: Recover the black magnetite precipitate by centrifugation or magnetic separation.
- Washing and Drying: Wash the precipitate several times with deionized water and acetone to remove residual salts and by-products. Dry the final product in an oven at 50-80 °C or via freeze-drying.
Diagram 1: Co-precipitation Experimental Workflow
Thermal Decomposition Method
The thermal decomposition method is a high-temperature approach that involves the pyrolysis of organometallic precursors (e.g., Fe(acac)₃, Fe(CO)₅, or metal acetylacetonates) in high-boiling-point organic solvents in the presence of stabilizing surfactants [25] [28]. This technique is renowned for producing MNPs with exceptional control over size and shape, high crystallinity, and narrow size distribution, making them ideal for precise biomedical applications [28] [27].
The process typically involves injecting a precursor solution into a hot solvent (e.g., octyl ether, benzyl ether) containing surfactants (e.g., oleic acid, oleylamine). The temperature, reaction time, precursor-to-surfactant ratio, and the specific choice of precursor and solvent collectively determine the final nanoparticle characteristics [28] [27]. A key advantage is the production of highly monodisperse nanoparticles with superior magnetic properties, such as high saturation magnetization [27]. A notable drawback is the complexity of the process and the frequent need for a subsequent phase transfer to make the particles water-dispersible for biological applications [28].
Table 2: Key Components and Their Roles in Thermal Decomposition
| Component | Function | Common Examples |
|---|---|---|
| Organometallic Precursor | Source of metal cations for nanoparticle formation. | Fe(acac)₃, Fe(CO)₅, metal acetylacetonates [28] [27]. |
| High-Boiling Solvent | Provides a medium for high-temperature reaction. | Octyl ether, benzyl ether, dioctyl ether [28]. |
| Stabilizing Surfactants | Control nucleation and growth; prevent agglomeration by coating particles. | Oleic acid, oleylamine [28] [27]. |
| Reaction Atmosphere | Often inert to prevent oxidation and control decomposition. | Nitrogen (N₂) or Argon (Ar) [28]. |
A standard protocol for the synthesis of monodisperse magnetite nanoparticles via thermal decomposition is as follows [28] [27]:
- Precursor Solution: Dissolve 2 mmol of iron(III) acetylacetonate (Fe(acac)₃) in a mixture of 20 mL of benzyl ether, 10 mmol of oleic acid, and 10 mmol of oleylamine.
- Decomposition and Reaction: Heat the mixture to 200-300 °C (typically around 265 °C) under a nitrogen atmosphere with vigorous magnetic stirring. Reflux for 30 minutes to 2 hours. The color change of the solution to black indicates the formation of magnetite nanoparticles.
- Cooling and Precipitation: Allow the reaction mixture to cool to room temperature. Add an excess of ethanol to precipitate the nanoparticles.
- Purification: Recover the nanoparticles by centrifugation. Re-disperse the precipitate in a non-polar solvent like hexane or toluene. This washing process can be repeated several times to remove excess surfactants and reaction by-products.
Diagram 2: Thermal Decomposition Experimental Workflow
Sol-Gel Method
The sol-gel method is a versatile, low-temperature chemical technique for fabricating metal oxide nanomaterials. The process involves the transition of a system from a liquid "sol" (colloidal suspension of solid particles in a liquid) into a solid "gel" phase [28]. For magnetite synthesis, iron alkoxide precursors are commonly hydrolyzed and condensed to form an iron oxide network [28].
The sol-gel route offers excellent control over the composition, porosity, and microstructure of the resulting material. It is particularly suited for creating thin films, coatings, and composite materials [28]. The main challenges associated with this method include the frequent need for post-synthesis thermal treatment (calcination) to achieve desired crystallinity and the potential for residual porosity or organic contamination. The magnetic properties of sol-gel-derived MNPs are highly dependent on the final heat treatment temperature and atmosphere [28].
A generalized experimental protocol is as follows:
- Sol Preparation: An iron precursor, such as an iron salt (e.g., FeCl₃) or an iron alkoxide (e.g., Fe(OC₂H₅)₃), is dissolved in a solvent (e.g., water or ethanol).
- Hydrolysis and Condensation: A catalyst (acid or base) is added to the solution to promote hydrolysis and polycondensation reactions, leading to the formation of a colloidal sol.
- Gelation: The sol is left to age, during which the colloidal particles link together to form a three-dimensional network, resulting in a gel.
- Drying and Calcination: The gel is dried to remove the solvent, resulting in a xerogel or aerogel. Subsequent calcination at elevated temperatures (e.g., 400-600 °C) is often performed to crystallize the amorphous iron oxide into magnetite or maghemite.
Comparative Analysis of Synthesis Methods
The choice of synthesis method is a critical determinant of the structural, magnetic, and application-specific properties of magnetic nanoparticles. The following table provides a direct comparison of the three methods discussed.
Table 3: Comprehensive Comparison of Advanced MNP Synthesis Methods
| Synthesis Method | Particle Size Range | Size Distribution | Crystallinity | Key Advantages | Key Limitations |
|---|---|---|---|---|---|
| Co-precipitation [29] [25] [28] | 2 - 15 nm | Broad to moderate | Moderate to High | Simple, fast, low cost, high yield, water-dispersible. | Broad size distribution, sensitivity to oxidation, control challenges. |
| Thermal Decomposition [28] [27] | 4 - 20 nm | Very Narrow (Monodisperse) | Very High | Excellent size/shape control, high crystallinity, superior magnetic properties. | Complex process, high cost, organic solvents, requires phase transfer. |
| Sol-Gel [28] | Wide range (highly tunable) | Broad | High (after calcination) | Good composition control, suitable for coatings/composites, low temperature. | Requires calcination, potential for impurities, can be porous. |
The Scientist's Toolkit: Essential Research Reagents and Materials
The synthesis and functionalization of MNPs require a suite of specialized reagents and materials. The following table details key items and their functions in the research process.
Table 4: Essential Research Reagent Solutions for MNP Synthesis
| Reagent/Material | Function in Synthesis | Specific Examples |
|---|---|---|
| Iron Salt Precursors | Source of Fe²⁺ and Fe³⁺ ions for nanoparticle nucleation and growth. | FeCl₂·4H₂O, FeCl₃·6H₂O [29] [31]. |
| Organometallic Precursors | Used in thermal decomposition for high-quality, monodisperse NPs. | Fe(acac)₃, Fe(CO)₅ [28] [27]. |
| Precipitating Agents (Bases) | Provide alkaline conditions necessary for precipitation of iron oxides. | NaOH, KOH, NH₃, Tetramethylammonium hydroxide (TEAOH) [29] [26] [31]. |
| Surfactants & Capping Agents | Control particle growth, prevent agglomeration, and provide surface functionality. | Oleic acid, oleylamine (thermal decomposition) [28] [27]; Dextran, PVA, Citrate (co-precipitation) [29] [25]. |
| Organic Solvents | Act as a reaction medium, particularly in thermal decomposition. | Benzyl ether, octyl ether, hexane, toluene [28]. |
| Inert Gases | Create an oxygen-free atmosphere to prevent oxidation of precursors and MNPs. | Nitrogen (N₂), Argon (Ar) [29] [26] [31]. |
The advanced synthesis of magnetic nanoparticles via co-precipitation, thermal decomposition, and sol-gel methods provides a powerful toolkit for materials scientists and drug development professionals. Each method offers a unique set of advantages and limitations, directly influencing the electrical, magnetic, and thermodynamic behaviors of the resulting nanomaterials. The continuous refinement of these synthesis protocols, coupled with a deeper understanding of structure-property relationships, is essential for driving innovation in nanomedicine, biosensing, and next-generation electronic devices. Future research will undoubtedly focus on enhancing the reproducibility, scalability, and biocompatibility of these synthesis routes, further unlocking the potential of magnetic nanoparticles in advanced technological applications.
Surface Functionalization and Coating Techniques for Enhanced Biocompatibility and Targeting
The performance of materials in biological environments is not solely determined by their bulk properties. The surface, serving as the primary interface with biological systems, dictates critical interactions with proteins, cells, and tissues. Surface functionalization and coating techniques are therefore pivotal in engineering materials for biomedical applications, from implantable devices to targeted drug delivery systems. These processes are designed to enhance biocompatibility, reduce immune rejection, and introduce targeting capabilities to guide materials to specific sites within the body [32] [33].
The efficacy of these surface modifications is deeply rooted in the electrical, magnetic, and thermodynamic behaviors of the underlying materials. The electronic structure influences protein adsorption and cell adhesion, magnetic properties can be harnessed for guided targeting, and thermodynamic stability ensures performance in the physiological environment. This whitepaper provides an in-depth technical guide to surface engineering strategies, framing them within the context of these fundamental material properties to offer researchers and scientists a comprehensive resource for advanced drug development and medical device design.
Fundamental Principles Linking Material Properties and Biological Response
The biological response to a material is a direct consequence of interfacial interactions, which are governed by the material's surface properties. Understanding the underlying physical principles is essential for rational design.
- Surface Energy and Thermodynamics: The thermodynamic principle of minimizing surface free energy drives the initial, spontaneous adsorption of proteins from biological fluids onto a material surface. Surfaces with high energy are generally more reactive and can lead to non-specific protein binding, potentially triggering undesirable immune responses. Coatings can modulate surface energy to control this "biofouling" phenomenon [32] [34].
- Electronic Structure and Charge: The electrical character of a surface, defined by its surface charge (zeta potential) and electronic band structure, affects electrostatic interactions with charged biological molecules. Surfaces with controlled charge can promote specific, desirable interactions with cell membranes or repel non-specific protein adsorption. For instance, tuning the work function and electron affinity through surface functionalization can alter how a material interacts with redox-active biological species [35].
- Magnetic Properties for Targeting: Incorporating materials with specific magnetic properties, such as high saturation magnetization and low coercivity, enables the external guidance of nanocarriers or implantable devices using magnetic fields. This is a core principle of magnetic targeting in drug delivery [36].
Surface Functionalization Techniques
Surface functionalization involves the introduction of chemical functional groups or biomolecules to a material's surface to impart new properties. These methods can be broadly classified into physical and chemical approaches.
Physical Functionalization Methods
Physical methods typically involve the deposition or physical adsorption of molecules without forming covalent bonds.
- Physical Vapor Deposition (PVD): PVD processes, such as sputtering and evaporation, are conducted under vacuum to deposit thin functional films onto a substrate. This technique is valuable for creating uniform coatings with controlled thickness and composition, often used for applying metallic or ceramic layers to implants [33] [34].
- Plasma Surface Treatment: This technique employs ionized gas (plasma) to activate a material's surface. By selecting specific plasma gases (e.g., O₂, N₂), various hydrophilic functional groups (–OH, –COOH, –NH₂) can be introduced, significantly increasing surface energy and improving cell adhesion. For example, O₂ and N₂ plasma treatment has been successfully used to functionalize poly(ε-caprolactone) (PCL) nanofibers [34].
Chemical Functionalization Methods
Chemical methods create stable, covalent linkages between the surface and the functionalizing molecules, offering enhanced durability.
- Cross-linking: This method uses bifunctional or multifunctional molecules to create bridges between surface functional groups and the desired ligand. Glutaraldehyde is a widely used cross-linker due to the high reactivity of its aldehyde groups toward amino and hydroxyl groups found in natural polymers. Other common chemical cross-linkers include carbodiimide and epichlorohydrin [34].
- Surface-Grafting: For polymers lacking reactive functional groups, surface-grafting can be employed. This often involves creating active sites on the polymer backbone (e.g., via plasma treatment) to initiate the polymerization of a second monomer directly from the surface. A documented example is the grafting of poly(acrylic acid) onto poly(ether sulfone) fibers, which can be further modified with amino or hydroxyl groups [34].
- Silane Coupling Agents: For silica-based and other hydroxyl-rich surfaces, silane chemistry is a standard technique. Silane agents form stable covalent siloxane linkages (Si-O-Si) with surface silanols, allowing the introduction of organic substituents such as amino, epoxy, or acrylate groups to enhance the interface between inorganic materials and biological systems [34].
Coating Techniques for Enhanced Biocompatibility
Advanced coating techniques create a physical barrier or a bioactive layer on a material, fundamentally altering its interaction with the biological environment.
Table 1: Overview of Common Biomedical Coating Techniques
| Technique | Principle | Applications | Key Advantages | Limitations |
|---|---|---|---|---|
| Chemical Vapor Deposition (CVD) | A precursor gas decomposes on a heated substrate, forming a solid coating. | Hydrophobic coatings, diamond-like carbon (DLC) films [33]. | Conformal, high-purity, and dense coatings. | High temperatures often required; complex precursors. |
| Plasma Spraying | A powder feedstock is injected into a plasma jet, melted, and accelerated onto a cool substrate. | Hydroxyapatite coatings on titanium orthopedic/dental implants [33] [37]. | High deposition rate; suitable for thick coatings. | Line-of-sight process; potential for porosity. |
| Sol-Gel Processing | A solution (sol) transitions into a gel network, which is then dried and solidified. | Bioactive glass and ceramic coatings [33]. | Low processing temperature; good compositional control. | Can be prone to cracking during drying. |
| Electrospinning | A high voltage is applied to a polymer solution to create fine, continuous fibers deposited as a non-woven mat. | Nanofibrous scaffolds for tissue engineering [33]. | High surface-area-to-volume ratio; mimics extracellular matrix. | Limited to polymer solutions/melts. |
| Vapor Deposition | A general term encompassing PVD and CVD for creating thin films from vapor phase. | Carbon-based nanostructured coatings [37]. | High purity and precise thickness control. | Requires vacuum systems; can be costly. |
Nanotechnology-Driven and Smart Coatings
Recent advancements have moved beyond passive coatings to dynamic, "smart" systems.
- Nanostructured Coatings: The integration of carbon-based nanomaterials (CNMs) like carbon nanotubes (CNT) and graphene is a significant advancement. These coatings are osteoconductive and highly biocompatible, allowing bone cells to come into close contact with the implant surface while inhibiting bacterial colonization [37].
- Stimuli-Responsive Coatings: These "smart" coatings react to changes in the local physiological environment (stimuli such as pH, temperature, or enzyme concentration) to provide on-demand therapeutic actions, such as controlled drug release [33].
- Biomimetic Coatings: These coatings are designed to closely replicate the natural tissue environment. They often incorporate extracellular matrix (ECM) components like collagen or peptides (e.g., RGD) to improve implant integration and reduce complications [33].
Experimental Protocols and Methodologies
This section provides detailed methodologies for key experiments cited in the literature, enabling replication and further development.
This protocol describes the conjugation of targeting antibodies to pre-formed polymersomes using a highly selective and biocompatible chemistry.
- Polymersome Formation and Amino-Functionalization: Assemble polymersomes from an amphiphilic block copolymer (e.g., poly(dimethylsiloxane)-block-poly(2-methyloxazoline)) using standard methods such as film rehydration or solvent shift. The hydrophilic corona must contain primary amine groups (-NH₂).
- Surface Amination Quantification: React the amino-functionalized polymersomes with a succinimidyl-activated fluorescent dye (e.g., NHS-fluorescein) in a suitable buffer (e.g., PBS, pH 8.5). Purify the conjugate via dialysis or size exclusion chromatography. Quantify the average number of amino groups per polymersome by comparing the fluorescence emission intensity to a standard curve of the free dye.
- Polymersome Activation (4FB Functionalization): Introduce 4-formylbenzoate (4FB) groups to the polymersome surface by reacting with the available amino groups using a heterobifunctional crosslinker like S-4FB. Purify the 4FB-functionalized polymersomes via dialysis.
- Ligand Modification (HyNic Functionalization): Functionalize the targeting ligand (e.g., an antibody or the enhanced Yellow Fluorescent Protein, eYFP) with 6-hydrazinonicotinate acetone hydrazone (HyNic) using its available amino groups.
- Conjugation and Purification: Mix the 4FB-functionalized polymersomes with the HyNic-functionalized ligand in aqueous buffer. The 4FB and HyNic groups will react to form a stable bond. Incubate for several hours at room temperature. Purify the resulting polymersome-ligand conjugates using tangential flow filtration or density gradient centrifugation to remove unreacted ligand.
- Validation and Testing:
- Use fluorescence spectroscopy or gel electrophoresis to confirm conjugation and determine the average number of ligands per polymersome (e.g., ~5 eYFP molecules per polymersome).
- Validate targeting specificity using antibiotin IgG conjugates on biotin-patterned surfaces or trastuzumab conjugates on breast cancer cells.
This general protocol outlines the steps for functionalizing various types of nanoparticles to improve biocompatibility and uptake.
- NP Synthesis and Characterization: Synthesize nanoparticles (e.g., silica, gold, metal oxides) and characterize their initial size, shape, and surface charge using Dynamic Light Scattering (DLS), Transmission Electron Microscopy (TEM), and zeta-potential analysis.
- Initial Surface Homogenization: Perform initial surface cleaning and homogenization to ensure a uniform starting point. For example, for nanodiamonds (NDs), this may involve oxidation to create a surface rich in a single functional group like carboxyls (-COOH) [34].
- Covalent Grafting:
- Silica NPs: React with aminosilanes (e.g., (3-aminopropyl)triethoxysilane, APS) to introduce amino groups (-NH₂) for subsequent bioconjugation [32].
- Gold NPs: Utilize thiol-carboxylic acids or other X-SH crosslinkers, which form covalent bonds with the gold surface, presenting a carboxylic acid (-COOH) group for further reaction [32].
- Metal Oxide NPs: Employ a ligand exchange strategy to substitute original surface groups with functional groups like diol, amine, carboxylic acid, or thiol [32].
- Carbon-based NPs: Generate -COOH groups via oxidation, which can then be activated for amide bond formation with amine-containing ligands [32].
- Ligand Coupling: Activate the introduced functional groups (e.g., using EDC/NHS chemistry for -COOH groups) to covalently couple targeting ligands such as antibodies, peptides, or aptamers.
- Biological Evaluation: Assess the success of functionalization by evaluating:
- Biocompatibility/Toxicity: Using cell viability assays (e.g., MTT assay) on relevant cell lines.
- Cellular Uptake: Using flow cytometry or confocal microscopy to quantify and visualize internalization by target cells.
Visualization of Workflows and Relationships
Surface Functionalization Decision Pathway
The following diagram outlines the logical decision-making process for selecting an appropriate surface functionalization strategy based on the base material and the desired biological outcome.
Experimental Workflow for NP Functionalization
This diagram illustrates the step-by-step experimental workflow for the functionalization and biological evaluation of nanoparticles, as detailed in Section 5.2.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 2: Key Reagent Solutions for Surface Functionalization Experiments
| Item | Function/Description | Example Application |
|---|---|---|
| Aminosilanes (e.g., APS) | Coupling agent that introduces primary amine (-NH₂) groups on silica and other hydroxylated surfaces. | Functionalization of silica nanoparticles for subsequent bioconjugation [32]. |
| Thiol-Carboxylic Acids (e.g., 16-Mercaptohexadecanoic acid) | Bifunctional linkers for noble metals. The thiol (-SH) group binds to gold/silver, presenting a carboxylic acid (-COOH) for further chemistry. | Creating a carboxylated surface on gold nanoparticles (AuNPs) [32]. |
| Glutaraldehyde | A homobifunctional crosslinker with aldehyde groups that react with primary amines. | Crosslinking amine-bearing surfaces to proteins or other amine-containing ligands [34]. |
| EDC & NHS (1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide & N-Hydroxysuccinimide) | A carbodiimide crosslinking system that activates carboxylic acid groups for efficient amide bond formation with amines. | Covalently attaching antibodies or peptides to carboxylated nanoparticle surfaces [32] [34]. |
| 4FB (4-Formylbenzoate) & HyNic (6-Hydrazinonicotinate) | A pair of heterobifunctional linkers that form a stable hydrazone bond. 4FB is used to functionalize the carrier, HyNic the ligand. | Biocompatible conjugation of antibodies to polymersomes for cell targeting [38]. |
| Heterobifunctional Crosslinkers (e.g., SMCC, Sulfo-SMCC) | Contain different reactive ends (e.g., NHS-ester and maleimide) for sequential conjugation of amines and thiols, reducing self-polymerization. | Site-specific conjugation of thiol-containing biomolecules to amine-functionalized surfaces. |
Surface functionalization and coating techniques represent a cornerstone of modern biomedical materials research. By strategically engineering the material-biology interface, it is possible to dictate biological outcomes, enhancing biocompatibility, enabling targeted delivery, and creating smart, responsive systems. The continued advancement of this field relies on a deep understanding of the interplay between the fundamental electrical, magnetic, and thermodynamic properties of materials and their surface chemistry. As new materials like Heusler alloys with their tailored electronic structures [35] and advanced carbon nanomaterials [37] emerge, the toolbox for surface engineering will only expand. Future progress will be driven by interdisciplinary research that combines novel material synthesis, sophisticated characterization, and rigorous biological testing to develop next-generation solutions for healthcare challenges.
Magnetic-Responsive Polymer Scaffolds and Hydrogels for Tissue Engineering
Magnetic-responsive polymer scaffolds and hydrogels represent a class of smart biomaterials that have gained significant traction in tissue engineering for their ability to be remotely controlled via external magnetic fields. These systems typically consist of hydrogel matrices embedded with magnetic nanoparticles (MNPs), combining the biocompatibility and structural similarity to native extracellular matrix of hydrogels with the responsive capabilities of magnetic components [39] [40]. The fundamental appeal of these materials lies in their ability to provide temporal and spatial control over biological processes, enabling precise manipulation of cell behavior, controlled drug delivery, and dynamic mechanical stimulation without invasive procedures [40].
From a thermodynamic perspective, these materials operate at the intersection of magnetic, electrical, and thermal energy domains. When subjected to alternating magnetic fields, magnetic nanoparticles within the hydrogel matrix can generate localized heat through mechanisms such as hysteresis losses or Néel relaxation, creating thermal gradients that influence both material properties and cellular activity [39]. Simultaneously, the arrangement of magnetic moments in response to applied fields represents a thermodynamic ordering process that can be described through fundamental equations relating magnetization, field strength, and temperature [41]. This interplay between magnetic responsiveness and thermodynamic principles enables the design of sophisticated materials capable of mimicking the dynamic nature of native tissues.
Fundamental Principles and Thermodynamic Foundations
Magnetic Thermodynamics in Biomaterials
The thermodynamic behavior of magnetic materials is governed by the interaction between magnetic moments and applied magnetic fields. In the context of tissue engineering scaffolds, this relationship can be described through fundamental thermodynamic equations that consider both the magnetic properties and their coupling to thermal effects. For a system of magnetic dipoles in a magnetic field, the energy is given by:
[E = -\sum{i=1}^{N} \vec{\mu}i \cdot \vec{H}]
where (\vec{\mu}_i) represents the individual magnetic dipole moments and (\vec{H}) is the applied magnetic field [41]. This alignment of dipoles against thermal agitation creates a competition between ordering energy and entropic effects, leading to temperature-dependent magnetic behavior characterized by the Curie temperature - the critical point where materials transition between ferromagnetic and paramagnetic states [41] [42].
The thermodynamic potentials for magnetic systems, particularly the Helmholtz free energy and Gibbs free energy, provide the foundation for understanding phase transitions in these materials. Statistical mechanics approaches, using partition functions, allow derivation of these potentials for different magnetic models, including the mean-field approximation which simplifies many-body interactions into an effective field [41]. This theoretical framework is essential for designing magnetic-responsive hydrogels with predictable thermal and mechanical behaviors under magnetic stimulation.
Material Response Mechanisms
Magnetic-responsive hydrogels exhibit several operational mechanisms when exposed to magnetic fields:
Deformation and Locomotion: Magnetic nanoparticles embedded within hydrogel networks experience forces and torques when subjected to magnetic field gradients, resulting in controlled deformation or movement of the scaffold [40]. This capability enables precise spatial manipulation for minimally invasive implantation and dynamic mechanical stimulation of encapsulated cells.
Thermogenesis: Under alternating magnetic fields, magnetic nanoparticles dissipate energy as heat through various loss mechanisms, enabling remote thermal activation of the scaffold [40]. This thermal response can be harnessed for controlled drug release, modulation of scaffold mechanical properties, or inducing thermal therapies.
Magnetic Mechanotransduction: The application of magnetic fields can exert mechanical forces on embedded nanoparticles, which then transmit these forces to attached cells or surrounding matrix, activating mechanosensitive signaling pathways [39]. This process mimics natural mechanical signaling in tissues, promoting differentiation and tissue formation.
Fabrication Methodologies
The synthesis of magnetic-responsive hydrogels employs several established techniques, each offering distinct advantages for controlling nanoparticle distribution and final material properties.
Primary Fabrication Approaches
Table 1: Magnetic Hydrogel Fabrication Methods
| Method | Process Description | Advantages | Limitations | Applications |
|---|---|---|---|---|
| Blending Method | Direct incorporation of pre-synthesized MNPs into hydrogel precursors before crosslinking | Simple procedure, good control over MNP concentration and properties | Potential nanoparticle aggregation, uneven distribution | Drug delivery systems, soft actuators [39] |
| In Situ Precipitation | Formation of MNPs within pre-formed hydrogel networks through sequential ion infusion and precipitation | Excellent nanoparticle distribution, strong particle-matrix integration | More complex process, limited control over crystal size | High-performance scaffolds for bone and neural tissue [43] |
| Grafting-onto Method | Chemical conjugation of pre-formed MNPs to polymer chains via covalent bonding | Prevents nanoparticle leakage, enhances mechanical stability | Requires functionalized nanoparticles and polymers, multi-step process | Long-term implantable scaffolds [39] |
Strategic Design Considerations
The fabrication of magnetic-responsive scaffolds requires careful consideration of multiple material parameters to achieve desired functionality:
Magnetic Nanoparticle Selection: The most commonly used magnetic materials include magnetite (Fe₃O₄), maghemite (γ-Fe₂O₃), and cobalt ferrite (CoFe₂O₄) nanoparticles, selected for their superparamagnetic properties and biocompatibility [39]. Surface functionalization with polymers or biological ligands enhances stability and prevents aggregation within hydrogel matrices.
Hydrogel Matrix Composition: Natural polymers such as chitosan, alginate, xanthan gum, and hyaluronic acid provide biocompatibility and biological recognition sites [43] [39]. Synthetic polymers including poly(vinyl alcohol), poly(ethylene glycol), and poly(N-isopropylacrylamide) offer precise control over mechanical properties and degradation profiles.
Crosslinking Strategies: Both physical (ionic, hydrogen bonding) and chemical (covalent) crosslinking approaches determine the mechanical integrity and responsiveness of the final construct. The crosslinking density must balance sufficient mechanical strength with appropriate mesh size for nutrient diffusion and cell migration.
Quantitative Performance Characteristics
Mechanical and Magnetic Properties
The incorporation of magnetic nanoparticles significantly alters the mechanical and functional properties of hydrogel scaffolds, with performance metrics varying based on composition and fabrication method.
Table 2: Performance Characteristics of Magnetic-Responsive Hydrogels
| Material Composition | MNP Type & Concentration | Storage Modulus (G') | Compressive Strength | Magnetic Response | Application Focus |
|---|---|---|---|---|---|
| Xanthan Gum/Chitosan [43] | Fe₃O₄ (concentration not specified) | Significant improvement over pristine PECH | Enhanced after MNP incorporation | Improved cell proliferation under magnetic field | Soft tissue engineering |
| PVA/HAP [39] | γ-Fe₂O₄ (0-80 wt%) | Not specified | Improved with m-nHAP addition | Linearly saturated magnetic strength | Bone tissue engineering |
| Bisphosphonate-modified HA [39] | Fe₃O₄ (2 w/v%) | Proper rheology | Not specified | Fast heat generation under AMF | Thermal therapy |
| Chitosan/PEG [39] | Fe₃O₄ (0-40 wt%) | Not specified | Not specified | Nanoheat generation | Drug delivery |
Biological Performance Metrics
The biological efficacy of magnetic-responsive scaffolds has been demonstrated through various in vitro and in vivo studies:
Cell Proliferation and Viability: NIH3T3 fibroblasts cultured on polysaccharide-based magnetic hydrogels showed significant improvements in cell proliferation and adhesion when exposed to an external magnetic field compared to non-magnetic controls [43]. This enhancement is attributed to both mechanical stimulation and possible effects on membrane receptor organization.
Tissue-Specific Differentiation: Magnetic stimulation has been shown to promote osteogenic differentiation of mesenchymal stem cells in bone tissue engineering applications, with increased expression of markers such as alkaline phosphatase and osteocalcin [39]. Similarly, in neural tissue engineering, magnetic scaffolds have demonstrated potential for guiding neurite extension and alignment.
Drug Release Kinetics: Magnetic thermoresponsive systems exhibit distinct release profiles under alternating magnetic fields, with studies demonstrating on-demand pulsatile release or sustained release patterns depending on the field application protocol [40]. This controlled release capability enables precise temporal delivery of growth factors or therapeutic agents.
Experimental Protocols and Methodologies
Fabrication Protocol:In SituPrecipitation Method
Based on the work with polysaccharide-based systems [43], the in situ precipitation method for fabricating magnetic-responsive hydrogels can be detailed as follows:
Reagents and Materials:
- Iron precursors: FeCl₂·4H₂O and FeCl₃·6H₂O in 1:2 molar ratio
- Polysaccharides: Chitosan and xanthan gum
- Crosslinking agent: D-(+)-glucuronic acid δ-lactone as green acidifying agent
- Ammonium hydroxide (25% NH₃ in water)
- Deionized water and buffer solutions as required
Procedure:
- Prepare separate solutions of chitosan (1-2% w/v in dilute acetic acid) and xanthan gum (1-2% w/v in deionized water).
- Mix the polysaccharide solutions in equal volumes under continuous stirring to form a polyelectrolyte complex base.
- Add iron salt solutions to the polymer mixture with vigorous stirring to ensure homogeneous distribution.
- Adjust pH to 10-11 using ammonium hydroxide solution to initiate precipitation of iron oxide nanoparticles.
- Add D-(+)-glucuronic acid δ-lactone gradually to lower pH and facilitate ionic crosslinking while controlling gelation kinetics.
- Allow the hydrogel to mature for 24 hours at room temperature before characterization.
- Wash the resulting magnetic hydrogel multiple times with deionized water to remove unreacted species and byproducts.
Characterization Methods:
- Microscopy: SEM analysis to confirm porous structure and nanoparticle distribution
- Spectroscopy: FTIR to verify chemical composition and interaction mechanisms
- Rheology: Oscillatory measurements to determine storage (G') and loss (G") moduli
- Magnetometry: VSM to quantify magnetic properties and responsiveness
Schematic of Magnetic Hydrogel Fabrication Process
Cell Culture and Magnetic Stimulation Protocol
Materials and Equipment:
- Magnetic hydrogel scaffolds (sterilized via UV or ethanol treatment)
- NIH3T3 fibroblasts or other relevant cell types
- Cell culture medium with serum
- External magnetic field setup (permanent magnets or electromagnetic system)
- Standard cell culture equipment (CO₂ incubator, biosafety cabinet)
Procedure:
- Sterilize magnetic hydrogel scaffolds (approximately 5mm diameter × 2mm thickness) using appropriate methods.
- Pre-condition scaffolds in culture medium for 24 hours before cell seeding.
- Seed cells at appropriate density (e.g., 50,000-100,000 cells/scaffold) and allow attachment for 4-6 hours.
- Apply external magnetic field using one of the following configurations:
- Static field: Permanent neodymium magnets with field strength of 50-500 mT
- Alternating field: Electromagnetic system with controlled frequency (50-500 kHz) and field strength
- Maintain cultures under standard conditions (37°C, 5% CO₂) with regular medium changes.
- Assess cell viability, proliferation, and morphology at predetermined time points using:
- Live/dead staining at day 1, 3, and 7
- MTT or Alamar Blue assay for metabolic activity
- Phalloidin/DAPI staining for cytoskeletal organization and nucleus morphology
- SEM analysis of cell-scaffold interactions
Magnetic Stimulation Experimental Workflow
Essential Research Reagents and Materials
The development and evaluation of magnetic-responsive scaffolds require specialized materials and characterization tools. The following table details key components for experimental work in this field.
Table 3: Essential Research Reagents for Magnetic-Responsive Scaffolds
| Category | Specific Items | Function/Purpose | Example Sources/Alternatives |
|---|---|---|---|
| Magnetic Components | Iron oxide nanoparticles (Fe₃O₄, γ-Fe₂O₃) | Provide magnetic responsiveness | Commercial suppliers (Sigma-Aldrich, NanoAmor) |
| Cobalt ferrite (CoFe₂O₄) nanoparticles | Alternative with higher magnetic moment | Laboratory synthesis via co-precipitation | |
| Polymer Matrices | Chitosan, Alginate, Xanthan Gum | Natural polymer base with biocompatibility | Various molecular weights and degrees of deacetylation |
| PVA, PEG, pNIPAM | Synthetic polymers with controllable properties | Different molecular weights and functional groups | |
| Crosslinking Agents | D-(+)-glucuronic acid δ-lactone | Green acidifying agent for ionic crosslinking | Specialty chemical suppliers |
| Glutaraldehyde, Genipin | Chemical crosslinkers for enhanced stability | Various concentrations and purity grades | |
| Characterization Tools | Vibrating Sample Magnetometer (VSM) | Quantification of magnetic properties | Commercial systems (LakeShore, Quantum Design) |
| Rheometer with magnetic attachment | Mechanical properties under magnetic fields | Temperature-controlled systems with magnetic options | |
| Cell Culture Components | NIH3T3 fibroblasts, Mesenchymal stem cells | Model systems for biocompatibility evaluation | ATCC and other biological repositories |
| Osteogenic, Chondrogenic differentiation media | Tissue-specific functionality assessment | Commercial kits or custom formulations |
Applications in Tissue Engineering
Bone Tissue Engineering
Magnetic-responsive scaffolds have shown significant promise in bone regeneration applications. The incorporation of hydroxyapatite-coated magnetic nanoparticles into polymer matrices such as poly(vinyl alcohol) creates scaffolds with improved compressive strength and osteoconductive properties [39]. When combined with magnetic stimulation, these constructs enhance osteogenic differentiation of mesenchymal stem cells through mechanotransduction pathways. The ability to remotely control scaffold properties also enables dynamic mechanical stimulation that mimics the natural loading environment of bone, further promoting matrix mineralization and bone formation.
Neural Tissue Engineering
In neural applications, magnetic hydrogels provide guidance cues for neurite extension and axonal regeneration. Studies have demonstrated that collagen-based magnetic scaffolds under static magnetic fields can promote aligned collagen fiber organization, creating contact guidance patterns for neural cells [39]. This aligned microstructure, combined with the ability to deliver neurotrophic factors in a controlled manner, addresses critical challenges in nerve repair, including directional growth and sustained biochemical support.
Cartilage and Soft Tissue Engineering
For cartilage tissue engineering, magnetic-responsive hydrogels offer solutions for creating zonally organized structures that mimic the native tissue architecture. Gradient-based magnetic hydrogels can be designed with varying mechanical properties through controlled nanoparticle distribution, replicating the transition from superficial to deep zones in articular cartilage [39]. Additionally, the dynamic mechanical stimulation provided by magnetic fields promotes chondrogenic matrix production and helps maintain the differentiated phenotype of chondrocytes in 3D culture.
Emerging Trends and Future Perspectives
The field of magnetic-responsive scaffolds continues to evolve with several emerging trends shaping future research directions:
Multimodal Responsive Systems: Integration of magnetic responsiveness with other stimuli-sensitive mechanisms (pH, temperature, light) creates sophisticated materials capable of complex behavior patterns [40]. These systems can respond to multiple environmental cues, providing enhanced control over scaffold properties and therapeutic actions in dynamically changing biological environments.
Advanced Manufacturing Techniques: The incorporation of magnetic materials into 3D bioprinting processes enables creation of complex, hierarchically organized structures with spatially controlled magnetic properties [44]. This approach allows precise patterning of magnetic nanoparticles within printed constructs, creating regional variations in mechanical properties and responsiveness.
Quantum Magnetic Effects: Emerging research on magnon-induced electric polarization in antiferromagnetic materials points to potential new mechanisms for coupling magnetic and electrical signaling in biological systems [12]. These fundamental discoveries could lead to novel scaffold designs that directly interface with native bioelectrical signaling pathways in tissues.
Personalized Therapeutic Platforms: The development of patient-specific magnetic scaffolds, guided by medical imaging and computational modeling, represents a growing trend toward personalized tissue engineering solutions. These approaches consider individual anatomical variations and biological needs for optimized regenerative outcomes.
The continued advancement of magnetic-responsive scaffolds requires interdisciplinary collaboration across materials science, physics, biology, and engineering. As understanding of magnetic-thermodynamic relationships in biological systems deepens, these smart materials will play an increasingly important role in addressing complex challenges in regenerative medicine and tissue engineering.
Magnetic Drug Targeting and Triggered Release Mechanisms for Chemotherapeutics and RNA
Cancer remains a major global health challenge, projected to reach 28.4 million cases annually by 2040 [45]. Conventional chemotherapy, while widely used, presents significant limitations including dose-dependent side effects and the development of multidrug resistance [45] [46]. Nanotechnology offers promising solutions, with Magnetic Nanoparticles (MNPs) emerging as powerful tools for targeted drug delivery. These systems leverage the unique magnetic properties of nanomaterials to enable spatiotemporal control over drug delivery and release, directly aligning with research into the electromagnetic and thermodynamic behaviors of advanced materials [45] [46] [7].
The fundamental principle of magnetic drug targeting involves functionalizing MNPs with therapeutic agents and guiding them to tumor sites using external magnetic fields, thereby increasing local drug concentration while minimizing systemic exposure [47] [48]. This approach can be further enhanced by designing MNPs that release their payload in response to specific triggers, such as pH changes, alternating magnetic fields (AMFs), or enzymatic activity [45] [49]. This review provides a comprehensive technical examination of MNP-based delivery systems for chemotherapeutics and RNA, detailing their classification, synthesis, functionalization, targeting mechanisms, and triggered release strategies, with a particular emphasis on the underlying material properties that enable these advanced functions.
Magnetic Nanomaterials: Classification and Synthesis
Classification of Magnetic Nanomaterials
MNPs can be broadly categorized based on their composition and magnetic properties. The following table summarizes the main classes and their key characteristics.
Table 1: Classification of Magnetic Nanomaterials for Drug Delivery
| Category | Core Material Examples | Key Magnetic Properties | Advantages | Limitations & Considerations |
|---|---|---|---|---|
| Magnetic Pure Metals | Fe, Co, Ni [45] | Ferromagnetic [45] | Excellent magnetic properties [45] | High reactivity and toxicity (Co, Ni); requires coating to prevent oxidation [45] |
| Magnetic Metal Oxides | Magnetite (Fe₃O₄), Maghemite (γ-Fe₂O₃) [45] | Superparamagnetic (below critical size) [45] [7] | High biocompatibility, low toxicity, approved for clinical use [45] [50] | Magnetic properties are highly dependent on synthesis method and size [45] |
| Multicomponent & Core/Shell NPs | Core: Fe₃O₄; Shell: SiO₂, Au, polymers [45] [7] | Tailorable magnetic core [45] | Multifunctionality (e.g., combined targeting, imaging, therapy); enhanced stability and biocompatibility [45] [49] | More complex synthesis; potential for heterogeneous structures [45] |
Superparamagnetism is a critical property for biomedical applications. Unlike ferromagnetic materials, which retain magnetization, superparamagnetic nanoparticles magnetize fully under an external magnetic field but exhibit no magnetic remanence once the field is removed [46] [7]. This prevents particle aggregation after removal of the targeting field and allows for their redispersion in the bloodstream [46].
Synthesis and Functionalization
The synthesis method dictates critical MNP properties such as size, crystallinity, and magnetic responsiveness [7].
- Chemical Methods: Co-precipitation is a common, simple method involving the precipitation of Fe²⁺ and Fe³⁺ ions in a basic aqueous solution, yielding iron oxide nanoparticles [45] [7]. Thermal decomposition of organometallic precursors in high-boiling-point organic solvents produces nanoparticles with high crystallinity and narrow size distribution [7]. The sol-gel method is used for coating magnetic cores with silica, enhancing stability and biocompatibility [7].
- Physical Methods: Laser ablation uses a high-energy laser to vaporize a bulk metal target in a liquid medium, producing nanoparticles with high purity and controlled morphology without by-products [7].
- Biological Methods: Employ microorganisms (e.g., Magneto spirillum species producing magnetosomes) or plant extracts to synthesize biocompatible MNPs [7].
Surface functionalization is crucial for creating effective drug delivery systems. Common strategies include:
- Polymer Coatings: Polyethylene glycol (PEG) improves stealth properties and circulation time [7].
- Ligand Conjugation: Targeting moieties like folate or transferrin receptors enable active targeting of cancer cells [47] [49].
- Stimuli-Responsive Linkers: pH-sensitive bonds (e.g., hydrazone) or thermosensitive polymers (e.g., poly(N-isopropylacrylamide)) allow for controlled drug release [45] [50].
Targeted Delivery Mechanisms
Magnetic targeting leverages the force exerted on MNPs by an external magnetic field gradient to overcome hydrodynamic forces in the bloodstream. The magnetic force ((Fm)) is given by: ( Fm = (χ{np} - χ{med}) V{np} (B \cdot \nabla)B / \mu0 ) where (χ{np}) and (χ{med}) are the magnetic susceptibilities of the nanoparticle and medium, (V{np}) is the nanoparticle volume, (B) is the magnetic flux density, and (\mu0) is the permeability of free space [47].
Two primary targeting strategies are employed:
- Passive Targeting: Relies on the Enhanced Permeability and Retention (EPR) effect, where the leaky vasculature and impaired lymphatic drainage of tumors allow nano-sized carriers (10-100 nm) to accumulate [45] [46].
- Active Targeting: Utilizes external magnetic fields to guide MNPs to the tumor site (magnetic targeting) and/or surface-functionalized ligands that bind to overexpressed receptors on cancer cells (biochemical targeting) [46] [47].
The following diagram illustrates the integrated workflow of magnetic drug targeting from administration to therapeutic action.
Diagram 1: Magnetic Drug Targeting Workflow
Triggered Release Mechanisms
A key advantage of MNP-based systems is the ability to control drug release remotely or in response to the tumor microenvironment.
- Magnetically Triggered Release: Alternating Magnetic Fields (AMF) can induce drug release through two main mechanisms:
- Magnetic Hyperthermia: AMF causes MNP oscillation, generating localized heat that can melt thermosensitive lipid bilayers (in magnetoliposomes) or cause conformational changes in thermoresponsive polymers, triggering drug release [46] [49].
- Magneto-Mechanical Actuation: High-frequency AMFs can induce physical vibrations or torque in MNPs, mechanically disrupting endosomal membranes or carrier structures to facilitate drug release without significant heating [45].
- Stimuli-Responsive Release Based on Tumor Microenvironment:
- pH-Triggered Release: The slightly acidic environment of tumor tissue (pH ~6.5-7.0) and endosomes/lysosomes (pH ~4.5-5.5) can cleave acid-labile linkers (e.g., hydrazone, acetal) connecting the drug to the MNP [45] [50].
- Enzyme-Triggered Release: Overexpressed enzymes (e.g., matrix metalloproteinases) in the tumor microenvironment can degrade specific peptide sequences used in the MNP coating or drug-linker, releasing the payload [47].
The thermodynamic and electromagnetic interactions underlying these release mechanisms are summarized below.
Diagram 2: Thermodynamic & Electromagnetic Release
Delivery of Chemotherapeutics and RNA
Chemotherapeutic Delivery
MNPs have been successfully conjugated with various chemotherapeutics. A prominent example is Doxorubicin (DOX), which is often attached via a pH-sensitive hydrazone bond [50]. In vivo studies in mouse models of breast cancer have demonstrated the superior efficacy of MNP-DOX conjugates. One study reported a significant inhibition of tumor growth in the MNP-DOX group compared to free DOX, alongside increased caspase-3 expression (apoptosis marker) and reduced Ki-67 expression (proliferation marker) [50]. Other drugs like cisplatin, methotrexate, and sorafenib have also been effectively delivered using MNPs, showing improved targeting and reduced off-target toxicity [45].
RNA-Based Therapy Delivery
Non-coding RNAs (e.g., siRNA, miRNA) offer a powerful approach for regulating gene expression in cancer cells but face challenges including low stability in serum and difficulty in crossing cell membranes [45]. MNPs can protect RNA from degradation and facilitate its delivery. The negatively charged RNA can be complexed with cationic polymers coated on MNPs or encapsulated within magnetic nanocarriers. Upon delivery and internalization, the RNA can silence target genes, such as oncogenes or genes involved in drug resistance [45].
Table 2: Quantitative In Vivo Efficacy of MNP-Delivered Therapeutics
| Therapeutic Agent | Disease Model | Key Experimental Findings | Mechanistic Insights |
|---|---|---|---|
| Doxorubicin (DOX) [50] | Mouse breast cancer (4T1 cells) | Tumor volume: 135 ± 28 mm³ (MNP-DOX) vs. significantly larger in control/DOX groups; ↑ caspase-3; ↓ Ki-67 [50] | pH-triggered DOX release; Enhanced apoptosis and reduced proliferation [50] |
| siRNA/miRNA [45] | Various cancer models | Regulation of gene transcription; Overcoming chemoresistance [45] | Protection of RNA from degradation; Magnetic field-enhanced cellular uptake [45] |
Experimental Protocols and Characterization
Detailed Synthesis and Conjugation Protocol (Based on Mouse Model Study)
The following protocol outlines the synthesis of DOX-conjugated MNPs and their subsequent in vivo evaluation [50].
- Synthesis of Iron Oxide MNPs:
- Reagents: FeCl₃·6H₂O, FeCl₂·4H₂O, ammonium hydroxide (28-30% w/w), deionized water, ethanol.
- Procedure: Dissolve FeCl₃·6H₂O and FeCl₂·4H₂O in deionized water at a 2:1 molar ratio. Heat the solution to 80°C under a nitrogen atmosphere with continuous stirring. Rapidly add ammonium hydroxide, resulting in a black precipitate. React for 30 minutes. Separate the precipitate using a magnet and wash with deionized water and ethanol. Dry under vacuum at room temperature for 24 hours [50].
- Surface Functionalization and Drug Loading:
- Reagents: (3-Aminopropyl)triethoxysilane (APTES), doxorubicin hydrochloride (DOX).
- Procedure: Disperse 1 g of MNPs in 100 mL of ethanol. Add 5 mL of APTES dropwise under stirring. Reflux the mixture at 80°C for 6 hours to yield amine-functionalized MNPs. Separate magnetically, wash with ethanol, and dry. To conjugate DOX, suspend 500 mg of amine-modified MNPs and 50 mg of DOX in 50 mL of PBS (pH 7.4). Stir gently at room temperature for 24 hours. Separate the MNP-DOX conjugates magnetically, wash with PBS to remove unbound drug, and lyophilize for storage. Drug loading efficiency is typically quantified by UV-Vis spectrophotometry of the supernatant [50].
- In Vivo Efficacy Evaluation:
- Animal Model: Female BALB/c mice inoculated with 4T1 breast cancer cells.
- Groups: Control, Free DOX, MNP only, MNP-DOX (n=6 per group).
- Dosing: Intravenous administration every 3 days for 2 weeks.
- Endpoint Measurements: Tumor volume and body weight are monitored regularly. Tumors are harvested for histological analysis (H&E staining) and immunohistochemical staining for Ki-67 and caspase-3 to assess proliferation and apoptosis, respectively [50].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Reagents for MNP Drug Delivery Research
| Reagent/Material | Function in Research | Specific Examples |
|---|---|---|
| Magnetic Cores | Provides superparamagnetic properties for targeting and hyperthermia. | Iron Oxide NPs (Fe₃O₄, γ-Fe₂O₃): Most common, high biocompatibility [45] [50]. |
| Co-precipitation Reagents | Simple, aqueous-phase synthesis of iron oxide NPs. | FeCl₃·6H₂O, FeCl₂·4H₂O: Iron precursors. NH₄OH: Precipitation agent [50]. |
| Surface Coating Agents | Enhance colloidal stability, prevent aggregation, improve biocompatibility. | Polyethylene Glycol (PEG): "Stealth" coating [7]. Silane Coupling Agents (e.g., APTES): Provide amine groups for further conjugation [50]. |
| Drug Linkers | Enable controlled, stimuli-responsive drug release. | Hydrazone Bond: pH-sensitive linker, cleaves in acidic tumor environment [50]. |
| Targeting Ligands | Mediate active targeting to cancer cells via specific receptor binding. | Folate: Targets overexpressed folate receptors on many cancer cells [49]. Antibodies: For specific antigen targeting (e.g., HER2) [49]. |
| Characterization Tools | Essential for analyzing NP properties. | TEM: Size and morphology [50]. XRD: Crystallinity [50]. VSM: Magnetic properties [50]. FTIR: Surface functionalization [50]. |
Magnetic nanoparticles represent a paradigm shift in oncological drug delivery, offering unprecedented control through the exploitation of their electromagnetic and thermodynamic properties. The ability to guide these nanocarriers externally and trigger drug release on demand addresses critical challenges in conventional chemotherapy, such as off-target toxicity and inadequate tumor accumulation. While significant progress has been demonstrated in preclinical models for both chemotherapeutic and RNA-based agents, the translation to clinical practice requires further optimization. Future efforts will focus on refining the synthesis of multifunctional, biocompatible MNPs, developing more powerful and focused magnetic field applicators, and conducting comprehensive safety and pharmacokinetic studies. The integration of MNP-based targeting with other modalities like hyperthermia and imaging (theranostics) promises to further personalize and improve cancer therapy outcomes.
Magneto-Mechanical Actuation and Hyperthermia as Anti-Cancer Strategies
Cancer therapy has long been challenged by the lack of selective interaction with neoplastic cells, leading to significant side effects and reduced treatment efficacy. Within this context, the exploration of the electrical, magnetic, and thermodynamic behaviors of materials has opened new frontiers in oncological treatment. Magneto-mechanical actuation and hyperthermia represent two emerging physical modalities that leverage the unique properties of magnetic nanomaterials to selectively target and destroy cancer cells with minimal impact on surrounding healthy tissues. These approaches exploit fundamental physics principles—specifically the interaction between magnetic materials and alternating magnetic fields—to generate localized therapeutic effects either through heat generation (hyperthermia) or mechanical force (actuation). The integration of these methods into a multimodal therapeutic framework demonstrates how advanced materials research can drive innovation in oncology, offering solutions to longstanding challenges in cancer treatment selectivity and efficacy. This whitepaper provides a comprehensive technical examination of these anti-cancer strategies, detailing their underlying mechanisms, experimental implementations, and synergistic potential for researchers and drug development professionals.
Fundamental Principles and Mechanisms
Magnetic Hyperthermia
Magnetic hyperthermia (MH) operates on the principle of converting electromagnetic energy into thermal energy through magnetic nanoparticles (MNPs) subjected to an alternating magnetic field (AMF). First proposed by Gilchrist et al. in 1957, this approach has evolved significantly with advancements in nanotechnology [51] [52]. The therapeutic effect occurs through several interconnected mechanisms:
Thermal Conversion: When subjected to an AMF (typically 50-400 kHz), MNPs dissipate energy as heat through hysteresis losses (for ferromagnetic/ferrimagnetic particles) or relaxation losses (for superparamagnetic particles) [51]. The specific heat absorption rate (SAR) or specific loss power (SLP) quantifies this thermal conversion efficiency, calculated as SAR = (c × mFe × ΔT/Δt) / m, where c is the volumetric specific heat capacity, mFe is the mass concentration of the magnetic element, and ΔT/Δt is the initial slope of temperature versus time [51].
Cellular Response: Elevated temperatures (41-46°C) induce apoptosis in cancer cells through multiple pathways including protein denaturation, DNA damage, and mitochondrial membrane potential depolarization [53]. Research demonstrates that hyperthermia at 43°C for 1 hour increases reactive oxygen species (ROS) and caspase-3 activation while triggering cytochrome c release and endoplasmic reticulum stress in human osteosarcoma cells [53].
Tumor Microenvironment Modulation: HT eliminates cancer stem cells (CSCs), sensitizes them to conventional treatments, and modulates the tumor microenvironment [54]. Additionally, hyperthermia recruits therapeutic mesenchymal stem cells (MSCs) for targeted delivery of antitumoral agents [54].
Magneto-Mechanical Actuation
Magneto-mechanical actuation (MMA) utilizes magnetic forces rather than thermal effects for cancer cell destruction. This approach employs magnetic nanoparticles subjected to alternating or rotating magnetic fields to generate mechanical stresses on cellular structures:
Direct Mechanical Destruction: The remote magnetic actuation of MNPs incubated with tumor cells triggers mechanical hits and vibrations that cause irreversible cellular damage [55]. Alternating magnetic fields constantly change the position of the MNPs depending on field gradients and frequency, generating torques onto cells [55].
Subcellular Targeting: Mechanical forces can be directed against specific cellular components. For instance, lysosome-accumulated MNPs under AMF can induce lysosomal cell death by increasing local temperature and enhancing reactive oxygen species production within these organelles [51].
Field-Induced Plasticity: The field-induced plasticity effect of magneto-sensitive materials enables shape-adaptable system parts that can be harnessed for biomedical applications [56]. This effect forms the basis for developing sophisticated actuator systems with tunable mechanical properties.
Table 1: Comparative Analysis of Hyperthermia and Magneto-Mechanical Actuation Mechanisms
| Parameter | Magnetic Hyperthermia | Magneto-Mechanical Actuation |
|---|---|---|
| Primary Mechanism | Heat generation via hysteresis/relaxation losses | Mechanical force/torque application |
| Field Parameters | Alternating magnetic field (50-400 kHz) | Alternating/rotating magnetic fields with gradients |
| Energy Conversion | Electromagnetic → Thermal energy | Electromagnetic → Mechanical energy |
| Cellular Effects | Protein denaturation, DNA damage, ROS production | Membrane disruption, cytoskeletal damage, organelle stress |
| Temperature Range | 41-46°C for therapeutic effect | No bulk temperature elevation required |
| Nanoparticle Types | SPIONs, magnetite, ferrites | Magnetic nanoparticles with high magnetization |
| Targeting Level | Tissue, cellular, subcellular | Cellular, subcellular, molecular |
Experimental Methodologies and Protocols
Nanoparticle Synthesis and Functionalization
Synthesis of Silver-Coated Magnetic Nanoparticles (MNP@Ag)
The synthesis of core-shell nanoparticles combines the magnetic properties of iron oxide with the additional therapeutic benefits of silver:
Magnetite Core Synthesis: Dissolve 1.1 g FeCl₂·4H₂O in 10 mL ultrapure water and filter through a 220 nm filter. Mix with 3 g FeCl₃·6H₂O and filter again. Add this solution to 400 mL of heated ultrapure water (90°C) under magnetic stirring (800 rpm). Quickly add 15 g solid NaOH, causing immediate color change to black. Continue stirring for 60 minutes after stopping heating [55].
Washing Procedure: Wash the magnetic nanoparticle suspension several times with ultrapure water until pH reaches approximately 6. Subject the obtained suspension (approximately 80 mL) to ultrasonication for 40 minutes using an ultrasonic homogenizer at 100% amplitude [55].
Silver Coating: Mix 40 mL MNP suspension (14.5 mg/mL) with 25 mL trisodium citrate (10 mg/mL), ultrasonicate for 5 minutes, and add to 400 mL heated ultrapure water (95°C) under magnetic stirring (1000 rpm). Quickly add 25 mL filtered AgNO₃ (200 mg) to the MNP-citrate suspension. Stir the suspension for 2 hours at 800 rpm maintaining temperature at approximately 95°C [55].
Surface Functionalization for Active Targeting
Active targeting strategies utilize cancer-specific ligands to enhance MNP accumulation in tumor cells:
Ligand Conjugation: Covalently attach bioreceptors including human epidermal growth factor, transferrin, folate, luteinizing hormone-releasing hormone, integrins, CD20, CD44, CD95, vascular endothelial growth factor, and CXCR4 to MNP surfaces [52].
Characterization Techniques: Employ transmission electron microscopy (TEM) for size and morphology analysis, dynamic light scattering for hydrodynamic diameter measurement, vibrating sample magnetometry for magnetic properties, and Fourier-transform infrared spectroscopy for surface chemistry verification [51] [57].
In Vitro Evaluation Protocols
Cell Viability Assessment Under Combined Treatment
Systematic analysis of combined hyperthermia and magneto-mechanical actuation:
Cell Culture: Plate dermal fibroblast cells and tumor cells (e.g., HeLa) in 96-well plates at 2 × 10⁴ cells/well and incubate for 48 hours in complete cell culture media (DMEM supplemented with 10% fetal bovine serum and 1% antibiotic-antimycotic) [55].
Nanoparticle Incubation: Add 10 µL of sterile silver-coated MNPs (concentration approximately 0.3 mg/mL) to each well and incubate for 24 hours [55].
Treatment Application: Expose cells to various configurations:
- Magnetic hyperthermia alone (alternating magnetic field)
- Magneto-mechanical actuation alone (alternating magnetic field with gradients)
- Chemotherapy alone (e.g., mitoxantrone)
- Combinations of the above modalities [55]
Viability Quantification: Perform MTT assay by adding 5-Dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide solution followed by dimethylsulfoxide. Measure absorbance at 570 nm and calculate cell viability as CV = 100 × (ODs - ODb)/(ODc - ODb), where ODs = OD of particle-treated cells, ODb = OD of blank, and ODc = OD of control cells [55].
Intracellular Temperature Mapping
Monitoring intracellular thermal responses during hyperthermia treatment:
GFP Transfection: Transfect cells with green fluorescent protein (GFP) bound to actin filaments to create an intracellular thermal reporter [58].
Fluorescence Lifetime Imaging: Utilize the temperature dependence of GFP's fluorescence lifetime parameter to sense intracellular temperature changes during hyperthermia treatment [58].
Calibration: Establish a calibration curve correlating fluorescence lifetime with temperature for accurate intracellular thermometry [58].
In Vivo Experimental Models
Animal Models: Utilize immunocompromised mice xenografted with human tumor cells (e.g., glioma, prostate cancer) for therapeutic efficacy studies [51].
Nanoparticle Administration: Administer MNPs via intratumoral injection or systemic delivery with targeting ligands to enhance tumor accumulation [57].
Magnetic Field Application: Apply alternating magnetic fields using specially designed coils with controlled frequency (50-400 kHz) and amplitude (H×f < 5×10⁹ A/m·s for safety) [51] [52].
Thermal Monitoring: Use infrared thermography or magnetic resonance thermometry to monitor temperature distribution in tumor and surrounding tissues [52].
Histological Analysis: Post-sacrifice, examine tumors and major organs for treatment effects, nanoparticle distribution, and potential toxicity [57].
Diagram 1: Experimental workflow for magneto-mechanical cancer therapy development
Quantitative Data and Therapeutic Efficacy
Hyperthermia Parameters and Outcomes
Table 2: Temperature-Dependent Cellular Responses to Hyperthermia
| Temperature | Exposure Time | Cell Line | Observed Effects | Molecular Mechanisms |
|---|---|---|---|---|
| 42°C | 4 hours | Melanoma (Me275, Me290) | Increased HSP70 and tumor antigen expression | Up-regulation of HSPs and tumor Ag expression [53] |
| 43°C | 40 minutes | Human tongue squamous cell carcinoma (Tca8113) | Apoptosis induction | Alteration of 107 proteins (57 significantly regulated) [53] |
| 43°C | 1 hour | Human osteosarcoma (U-2) | Apoptosis via ROS increase | Caspase-3 activation, cytochrome c release, ER stress [53] |
| 43°C | 1 hour | Spermatocytes (in vivo) | Deregulated RNA metabolism | piRNA metabolism disruption [53] |
| 42°C | 1 hour | Cervical carcinoma (SiHa, HeLa) | p53-dependent apoptosis, G2-phase arrest | E6 degradation enabling p53 activation [53] |
| 45°C | 30 minutes | Melanoma (B16-F10) | G0/G1 cell cycle arrest | Cell cycle progression inhibition [53] |
| 50-70°C | Not specified | Glioma (in vivo) | Necrosis and apoptosis | Direct thermal destruction [53] |
Combination Therapy Efficacy
Research demonstrates significantly enhanced efficacy when magneto-mechanical approaches are combined with other treatment modalities:
Triple Combination Superiority: The combination of hyperthermia, magneto-mechanical actuation of silver-coated magnetite nanoparticles (MNP@Ag), and chemotherapy (mitoxantrone) was the only configuration that satisfied both safety for normal cells (fibroblasts) and high cytotoxicity for tumor cells (HeLa), reducing HeLa viability to approximately 32% while maintaining fibroblast viability at 80% [55].
Synergistic Effects: The cytotoxic effect of combined treatments is not merely additive but results from "a nonlinear conjugation of the triggers in a dynamic regime imposed by the magneto-mechanical actuation of the nanoparticles" [55]. This synergy enables substantial reduction of chemotherapeutic drug doses while maintaining therapeutic performance.
Nanoparticle-Mediated Sensitization: MNPs-MH enhances the sensitivity of cancer cells to traditional treatments including chemotherapy, radiotherapy, immunotherapy, photothermal/photodynamic therapy, and gene therapy [51]. For instance, localized induction heat from MNPs can disrupt subcellular structures like lysosomes, leading to enhanced reactive oxygen species production and cell death [51].
Table 3: Multimodal Therapy Efficacy Comparison
| Treatment Configuration | HeLa Viability (%) | Fibroblast Viability (%) | Therapeutic Index |
|---|---|---|---|
| Control (no treatment) | 100 | 100 | 1.0 |
| Chemotherapy alone | 75 | 85 | 1.1 |
| Hyperthermia alone | 65 | 90 | 1.4 |
| Magneto-mechanical actuation alone | 70 | 88 | 1.3 |
| Hyperthermia + Chemotherapy | 55 | 82 | 1.5 |
| Magneto-mechanical + Chemotherapy | 60 | 80 | 1.3 |
| Hyperthermia + Magneto-mechanical | 50 | 85 | 1.7 |
| All three modalities combined | 32 | 80 | 2.5 |
Therapeutic Index = Fibroblast Viability / HeLa Viability [55]
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 4: Essential Research Reagents for Magneto-Mechanical Cancer Therapy Studies
| Reagent/Material | Specifications | Research Application | Key Considerations |
|---|---|---|---|
| Magnetic Nanoparticles | SPIONs (10-100 nm), magnetite, maghemite | Core therapeutic agents for hyperthermia and actuation | Size, composition, shape, surface chemistry affect heating efficiency and biocompatibility [51] [57] |
| Coating Materials | Silver, gold, silica, PAA, PEG, citrates | Improve biocompatibility, add functionality | Silver coating provides additional antitumor effects; PEG extends circulation time [55] [57] |
| Targeting Ligands | Folate, transferrin, EGFR, LHRH, integrins | Active tumor targeting | Enhance specific cellular uptake via receptor-mediated endocytosis [52] |
| Cell Lines | HeLa, MCF-7, U-87, PC3, patient-derived cells | In vitro efficacy and safety assessment | Choose relevant cancer types with appropriate characteristics [53] [55] |
| Animal Models | Immunocompromised mice, rat glioma models | In vivo therapeutic evaluation | Orthotopic models may better replicate tumor microenvironment [53] |
| Alternating Magnetic Field Generators | Custom coils (100-400 kHz), commercial systems (NanoScale, MagForce) | Application of activating magnetic fields | Frequency and amplitude must satisfy safety criteria (H×f < 5×10⁹ A/m·s) [51] [52] |
| Characterization Equipment | VSM, TEM, DLS, FTIR, spectrophotometers | Nanoparticle characterization and analysis | Comprehensive characterization essential for reproducible research [51] [57] |
| Viability Assays | MTT, WST, calcein-AM/propidium iodide | Assessment of treatment efficacy and toxicity | Multiple assays recommended for confirmation [53] [55] |
Diagram 2: Cellular response pathways to combined magneto-mechanical therapy
Magneto-mechanical actuation and hyperthermia represent promising anti-cancer strategies that leverage fundamental principles from electrical, magnetic, and thermodynamic behavior of materials research. The integration of these physical approaches with conventional chemotherapy creates synergistic effects that enhance tumor cell destruction while potentially reducing required drug doses. The experimental methodologies outlined provide researchers with robust protocols for synthesizing functional magnetic nanoparticles, evaluating their efficacy through in vitro and in vivo models, and assessing safety profiles. As these technologies advance, key challenges remain in optimizing nanoparticle designs for enhanced targeting and energy conversion efficiency, developing more sophisticated magnetic field application systems, and conducting comprehensive safety assessments of novel nanomaterials. The continued collaboration between materials scientists, physicists, and oncologists will be essential to translate these promising approaches from laboratory research to clinical applications, ultimately offering new hope for cancer patients through more selective and effective treatment options.
Overcoming Biocompatibility, Stability, and Manufacturing Hurdles
Mitigating Cytotoxicity and Reactive Oxygen Species Generation in Magnetic Nanoparticles
Magnetic nanoparticles (MNPs) have emerged as powerful tools in biomedicine, enabling advances in targeted drug delivery, magnetic hyperthermia, diagnostic imaging, and theranostic applications [45] [7]. Their distinctive feature is the responsiveness to external magnetic fields, which allows for precise spatial control within biological systems. However, the translation of MNP-based technologies to clinical applications faces a significant challenge: the inherent and potential cytotoxicity, often linked to the generation of reactive oxygen species (ROS) [59]. Understanding and mitigating these adverse effects requires a fundamental perspective that frames MNP behavior within the context of electrical, magnetic, and thermodynamic principles.
At the nanoscale, material properties diverge significantly from their bulk counterparts. Finite-size effects and the dramatically increased surface-to-volume ratio dictate novel magnetic behaviors, most notably superparamagnetism, where nanoparticles exhibit a magnetically responsive state without retained magnetization upon removal of the external field [60]. Simultaneously, the thermodynamic stability of these systems is size-dependent; key parameters such as cohesive energy, magnetic anisotropy energy, and the energy barrier to moment reversal all decrease with reduced particle size [61]. This lowered energy landscape makes MNPs susceptible to thermal fluctuations and underlies their propensity to participate in redox chemistry, potentially catalyzing ROS generation via Fenton and Haber-Weiss reactions [59]. Therefore, a rational strategy to mitigate MNP cytotoxicity must be grounded in a thorough understanding of these unique nanoscale thermodynamic and magnetic behaviors.
Theoretical Foundations: Linking Magnetic, Thermodynamic, and Electrical Behaviors to Biological Effects
Magnetic and Thermodynamic Properties at the Nanoscale
The magnetic behavior of nanomaterials is governed by a delicate balance of energy terms. For a spherical, single-domain MNP, the magnetic anisotropy energy barrier is given by ( Ea = K{eff}V ), where ( K{eff} ) is the effective anisotropy constant and ( V ) is the particle volume [60]. This energy barrier stabilizes the magnetic moment along an easy axis. However, as the particle size decreases, ( Ea ) becomes comparable to the thermal energy ( kBT ) (where ( kB ) is Boltzmann's constant and ( T ) is temperature). When ( Ea \ll kBT ), the magnetic moment fluctuates rapidly, resulting in the superparamagnetic state, characterized by zero coercivity and remanence [60]. This state is highly desirable for biomedical applications as it prevents particle aggregation after removal of the magnetic field and enables redispersion.
The thermodynamic properties of MNPs are inherently size-dependent. The cohesive energy, which quantifies the thermal stability of a material, decreases as the particle size is reduced due to the increased proportion of surface atoms with dangling bonds [61]. This decreased cohesive energy and the associated lower melting temperature can enhance the surface reactivity of MNPs, making them more prone to oxidation and ion leaching, which are primary pathways for ROS generation [59] [61]. Furthermore, the magnetic order in nanoparticles is also thermodynamically constrained; the Curie temperature (( T_C )), the critical point for ferromagnetic-to-paramagnetic transition, decreases with reducing particle size, influencing their performance in applications like magnetic hyperthermia [61].
Reactive Oxygen Species (ROS) Generation Pathways
ROS, such as hydroxyl radicals (•OH), superoxide anions (O₂•⁻), and hydrogen peroxide (H₂O₂), are oxygen-containing molecules with high chemical reactivity. At normal physiological levels, ROS play a role in cellular signaling, but elevated levels cause oxidative stress, damaging proteins, lipids, and DNA, ultimately leading to apoptosis or other forms of cell death [59].
MNPs, particularly those containing iron, can catalyze ROS generation through two primary mechanisms:
- Fenton Reaction: ( Fe^{2+} + H2O2 \rightarrow Fe^{3+} + \bullet OH + OH^- )
- Haber-Weiss Reaction: ( O2^{\bullet-} + H2O2 \rightarrow O2 + \bullet OH + OH^- ) (catalyzed by trace metals)
The surface of MNPs acts as a platform for these reactions. The high surface energy, a thermodynamic driver, and the presence of transition metal ions in multiple oxidation states (e.g., Fe²⁺/Fe³⁺) facilitate electron transfer processes, converting the less reactive H₂O₂ into the highly damaging •OH radical [59]. This catalytic activity is directly influenced by the MNP's size, crystal structure, and surface chemistry, all of which are controllable through synthesis and functionalization.
The following diagram illustrates the interconnected thermodynamic, magnetic, and electronic properties of MNPs and how they drive the biological response of ROS generation and cytotoxicity.
Quantitative Data on MNP Properties and Cytotoxicity
The relationship between the physical properties of MNPs and their biological impact can be quantified. The following tables summarize key data from the literature, providing a resource for understanding how characteristics like size, coating, and composition influence cytotoxicity and ROS generation.
Table 1: Impact of MNP Core Properties on Cytotoxicity and ROS Generation
| Material/ Composition | Size Range (nm) | Saturation Magnetization (emu/g) | Key Findings on Cytotoxicity/ROS | Reference |
|---|---|---|---|---|
| Magnetite (Fe₃O₄) | 4 - 20 nm | ~85 (40 nm) | Size-dependent ROS; apoptosis in A549 cells via oxidative stress; lower Ms than bulk. | [59] [7] [60] |
| Maghemite (γ-Fe₂O₃) | 10 - 30 nm | N/A | Surface oxidation state influences Fenton reactivity and ROS generation potential. | [7] [60] |
| Cobalt (Co) Ferrite | 15 - 40 nm | N/A | Higher anisotropy can increase magnetic response but Co ions may elevate toxicity. | [7] |
| Iron Pure Metal | < 50 nm | N/A | Excellent magnetic properties but high reactivity and rapid oxidation necessitate coatings. | [45] [7] |
| Polymer Microcapsules (with packed MNPs) | ~3000 nm (3 µm) | N/A | Enhanced localized cytotoxicity under AMF without bulk heating; superior to free MNPs. | [62] |
Table 2: Effectiveness of Different Mitigation Strategies
| Mitigation Strategy | Example Materials/ Methods | Key Outcomes & Reduction in Toxicity | Reference |
|---|---|---|---|
| Biocompatible Polymer Coating | PEG, Dextran, Polyethyleneimine | Enhanced colloidal stability, reduced immune recognition, and minimized nonspecific interactions. | [45] [7] |
| Inorganic Coating | Silica (SiO₂), Gold (Au) | Creates physical barrier preventing ion leaching; Au provides excellent biocompatibility. | [7] |
| Surface Functionalization with Antioxidants | N-acetylcysteine (NAC), Cerium Oxide | ROS scavenging directly at particle surface; CeO₂ acts as a catalytic antioxidant. | [59] [63] |
| Ligand Targeting | Folate, CREKA peptide, RGD | Enhances specific cellular uptake, reducing required dose and off-target effects. | [45] [7] |
| Structural Modification (Core-Shell) | FePt-Fe₃O₄, Fe₃O₄@TiO₂ | Combines high magnetic moment with stable, less-reactive shell. | [59] [7] |
Experimental Protocols for Assessing and Mitigating Cytotoxicity and ROS
To systematically evaluate the safety and efficacy of engineered MNPs, standardized experimental protocols are essential. The following sections detail key methodologies for assessing ROS generation and cytotoxicity, which are critical for validating any mitigation strategy.
Protocol 1: Standard In Vitro ROS Detection Assay
Objective: To quantify the generation of reactive oxygen species induced by MNPs in a cell culture model.
Materials:
- DCFH-DA Assay Kit: (2',7'-Dichlorofluorescin diacetate) Cell-permeable dye that becomes fluorescent upon oxidation by ROS.
- Cell Line: Adherent cells such as human lung epithelial cells (A549) or human liver cells (HepG2) [59].
- MNPs: Suspensions of test and control MNPs in sterile PBS or cell culture medium.
- Positive Control: Tert-butyl hydrogen peroxide (t-BOOH).
- Microplate Reader: For fluorescence measurement (Excitation ~485 nm, Emission ~535 nm).
Procedure:
- Cell Seeding: Seed cells in a 96-well plate at a density of 1 x 10⁴ cells per well and culture for 24 hours to allow adhesion.
- Dye Loading: Remove the medium and incubate cells with 100 µM DCFH-DA in serum-free medium for 30-60 minutes at 37°C.
- MNP Exposure: Carefully wash the cells to remove excess dye. Treat with a range of MNP concentrations (e.g., 0-200 µg/mL) dispersed in fresh culture medium. Include a negative control (medium only) and a positive control (t-BOOH).
- Incubation and Measurement: Incubate the plate for a predetermined period (e.g., 4-24 hours). Measure the fluorescence intensity at regular intervals using a microplate reader.
- Data Analysis: Normalize fluorescence data to cell viability (e.g., via MTT assay). Express results as fold-increase in fluorescence relative to the negative control.
Protocol 2: Cytotoxicity Evaluation via MTT Assay
Objective: To determine the metabolic activity and viability of cells after exposure to MNPs.
Materials:
- MTT Reagent: (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) Yellow tetrazole, reduced to purple formazan by living cells.
- Cell Line and MNPs: As described in Protocol 1.
- Dimethyl Sulfoxide (DMSO): For solubilizing formazan crystals.
- Microplate Reader: For measuring absorbance at 570 nm.
Procedure:
- Cell Seeding and Exposure: Seed cells in a 96-well plate and expose to MNPs as described in Steps 1 and 3 of Protocol 1.
- MTT Incubation: After the exposure period, add MTT reagent (0.5 mg/mL final concentration) to each well and incubate for 2-4 hours at 37°C.
- Solubilization: Carefully remove the medium containing MTT and add DMSO to each well to dissolve the formed formazan crystals.
- Absorbance Measurement: Gently shake the plate and measure the absorbance at 570 nm, with a reference wavelength of 630-650 nm.
- Data Analysis: Calculate the percentage of cell viability relative to the untreated control cells. The IC₅₀ value (concentration that inhibits 50% of metabolic activity) can be determined from dose-response curves.
The workflow for a comprehensive safety assessment of engineered MNPs, integrating the protocols above, is visualized below.
The Scientist's Toolkit: Essential Reagents and Materials
Table 3: Research Reagent Solutions for MNP Cytotoxicity and ROS Studies
| Category | Item | Specific Function & Rationale |
|---|---|---|
| Synthesis & Coating | Iron(III) Chloride (FeCl₃) & Iron(II) Sulfate (FeSO₄) | Common precursors for co-precipitation synthesis of iron oxide MNPs (e.g., magnetite) [7]. |
| Polyethylene Glycol (PEG)-Silane | Bifunctional molecule for creating stealth, biocompatible coatings that reduce protein fouling and improve stability [45] [7]. | |
| Tetramethylorthosilicate (TMOS) | Precursor for creating a dense, inert silica shell around MNPs via the sol-gel method, inhibiting ion leaching [7]. | |
| ROS & Cytotoxicity Assays | DCFH-DA Probe | Cell-permeable chemical probe that becomes fluorescent upon oxidation by a broad range of ROS, enabling quantitative measurement [59]. |
| N-Acetylcysteine (NAC) | Broad-spectrum antioxidant and ROS scavenger; used both as a surface functionalization agent and a positive control in inhibition experiments [59] [63]. | |
| MTT Assay Kit | Standard colorimetric assay for measuring the metabolic activity of cells, a proxy for cell viability and proliferation after MNP exposure. | |
| Cell Culture & Analysis | A549 Cell Line | Human lung adenocarcinoma epithelial cell line, commonly used as a model for pulmonary toxicity studies of nanoparticles [59]. |
| HepG2 Cell Line | Human hepatocellular carcinoma cell line, a standard model for assessing nanomaterial-induced liver toxicity [59]. | |
| Apocynin | An inhibitor of NADPH oxidase assembly; used to investigate the contribution of cellular enzymatic sources to overall ROS levels [63]. |
Advanced Strategies: Beyond Surface Coating
While surface coatings are a primary defense, advanced material engineering strategies offer more sophisticated control over MNP biocompatibility.
Magnetic Microcapsules: An innovative approach involves packing high concentrations of MNPs into polyelectrolyte microcapsules (≈3 µm diameter). These capsules can be internalized by cells, and upon application of an alternating magnetic field (AMF), they produce a potent localized cytotoxic effect without a measurable temperature increase in the bulk medium. This strategy confines the magnetic activity and associated stress to the intracellular compartment, enhancing therapeutic specificity and minimizing off-target effects [62].
Exploiting ROS for Therapy: It is important to note that MNP-induced ROS generation is not solely a detriment. This capability can be harnessed therapeutically. In cancer treatment, the elevated ROS levels in tumor cells can be exploited to achieve selective killing. MNPs can be designed to amplify ROS stress specifically in cancer cells, overwhelming their already compromised antioxidant defenses and triggering apoptosis [59]. This represents a paradigm where understanding and controlling the mechanism allows a potential liability to be transformed into a therapeutic asset.
The journey toward safe and effective magnetic nanoparticles in biomedicine is inextricably linked to a deep understanding of their fundamental electrical, magnetic, and thermodynamic behaviors. The mitigation of cytotoxicity and ROS generation is not merely a problem of biological compatibility but a materials science challenge rooted in nanoscale phenomena. Strategies such as sophisticated surface engineering, the use of antioxidant coatings, and the development of novel composite structures like magnetic microcapsules have shown significant promise. By continuing to design MNPs with a rational consideration of their size-dependent magnetic stability, surface energy, and catalytic potential, researchers can effectively suppress adverse cellular responses while preserving and even enhancing their diagnostic and therapeutic capabilities. Future work will likely focus on "smart" MNPs that can dynamically respond to the specific microenvironment of diseased tissues, offering unparalleled precision in biomedical applications.
Optimizing Magnetic Properties via Doping, Core-Shell Structures, and Crystallinity Control
The pursuit of advanced magnetic materials is a cornerstone of modern technology, influencing sectors ranging from information storage and spintronics to biomedical applications like magnetically guided drug delivery. The electrical, magnetic, and thermodynamic behaviors of materials are deeply intertwined, dictating performance in operational environments. This whitepaper examines three principal strategies for optimizing magnetic properties—doping, core-shell structuring, and crystallinity control—framed within the context of their broader impact on material behavior. These approaches enable precise tuning of magnetic characteristics such as saturation magnetization, coercivity, and blocking temperature by manipulating composition, interface interactions, and structural order. The following sections provide a technical guide for researchers and scientists, detailing the mechanisms, experimental data, and methodologies underpinning these advanced material design strategies.
Magnetic Property Enhancement Through Doping
Doping involves the intentional introduction of specific elements into a host material to alter its electronic structure and magnetic interactions. This strategy is particularly effective in perovskite and metal oxide systems, where the substitution of cations can induce or enhance magnetic moments and modify magnetic ordering.
Mechanisms and Material Systems
In perovskite materials with the general formula ABO₃, the magnetic properties are primarily governed by the B-site transition metal cations. Doping at either the A- or B-site can dramatically change the superexchange interactions between metal ions, influencing the overall magnetic behavior [11]. For instance, introducing rare-earth elements at the A-site or transition metals at the B-site can lead to phenomena such as ferromagnetism, antiferromagnetism, or spin-glass behavior [11].
A prominent example is titanium dioxide (TiO₂), a typically diamagnetic material. Research shows that doping TiO₂ with elements like iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), molybdenum (Mo), or rare-earth metals such as cerium (Ce), erbium (Er), neodymium (Nd), and gadolinium (Gd) can induce room-temperature ferromagnetism [64]. The magnetic outcome is highly dependent on the dopant type, concentration, and synthesis method. For instance, Ni and Cu doping induces ferromagnetism in TiO₂ nanoparticles, though with weak saturation magnetization that can be improved by increasing dopant concentration or optimizing the synthesis process [64]. Conversely, one study found that Fe-doping of TiO₂ nanoparticles unexpectedly resulted in the highest saturation magnetization for the undoped sample, attributed to its larger crystallite size, highlighting that saturation magnetization is strongly dependent on crystallite size [64]. Lanthanum (La) doping modulates the concentration of oxygen vacancies, which in turn modulates room-temperature magnetism without disturbing the anatase phase's tetragonal structure [64].
Quantitative Effects of Doping
Table 1: Magnetic Properties of Doped Titanium Dioxide (TiO₂) Nanoparticles
| Dopant Element | Doping Concentration | Observed Magnetic Behavior | Key Findings |
|---|---|---|---|
| Iron (Fe) [64] | Varied (e.g., 1%, 3%, 5%) | Ferromagnetism (varies) | Saturation magnetization can decrease with increasing Fe concentration; linked to crystallite size. |
| Cobalt (Co) [64] | Different percentages | Anti-ferromagnetism | Observed at low temperatures; Curie-Weiss temperature increases with dopant concentration. |
| Nickel (Ni) [64] | Not Specified | Induced Ferromagnetism | Weak saturation magnetization values were obtained. |
| Molybdenum (Mo) [64] | Not Specified | Room-Temperature Ferromagnetism | Confirmed in doped TiO₂ NPs. |
| Neodymium (Nd) [64] | Not Specified | Unsaturated Ferromagnetism | Observed at high fields due to unpaired electron pairs from rare earth metals. |
| Gadolinium (Gd) [64] | Not Specified | Paramagnetism | Transformation from weak ferromagnetism of pure TiO₂. |
Core-Shell Nanostructures for Magnetic Optimization
Core-shell nanostructures represent a powerful architecture for creating composite magnetic materials with synergistic properties. These structures consist of a nanoparticle core encapsulated within a shell of a different material, allowing for interface-driven magnetic effects such as exchange bias and enhanced spin coupling.
Architecture and Magnetic Coupling
Core-shell structures can be configured in various ways, including conventional (ferromagnetic core/antiferromagnetic shell), inverted (antiferromagnetic core/ferromagnetic shell), and more complex multi-shell configurations [65] [66]. The interface between the core and shell is critical, as it facilitates magnetic exchange coupling. This coupling can pin the magnetization of the ferromagnetic layer, leading to the exchange bias effect—a horizontal shift in the hysteresis loop—which is vital for enhancing the thermal stability of magnetic nanostructures [65].
A significant enhancement was demonstrated in Co-oxide/Co/Co-oxide core/shell/shell nanostructures, where the extra exchange coupling at the additional shell-shell interface led to a remarkable increase in coercivity (by three orders of magnitude) and exchange bias strength (by four orders of magnitude) compared to simple Co-oxide/Co core/shell structures [65]. This shows that multiple interfaces can synergistically enhance magnetic properties.
In bi-magnetic systems, such as ZnFe₂O₄@NiFe₂O₄ (zinc ferrite core with a nickel ferrite shell), the formation of a coherent interface improves net crystallinity and spin coupling [66]. This results in stronger overall magnetism, evidenced by higher saturation magnetization and a doubling of the blocking temperature compared to the ZnFe₂O₄ cores alone [66]. The blocking temperature is the temperature above which superparamagnetic behavior occurs, and its increase indicates greater magnetic stability.
Impact of Shell Thickness
The thickness of the shell is a critical parameter in determining magnetic properties. In the Co-oxide/Co/Co-oxide system, the strongest exchange bias was achieved with the thinnest outer Co-oxide shell [65]. Furthermore, a non-monotonic, oscillating behavior of the exchange bias was observed with increasing shell thickness, attributed to the dependence on antiferromagnetic outer shell thickness variation at the expense of the simultaneous opposite variation in the ferromagnetic inner shell [65]. This complex relationship underscores the need for precise control over shell dimensions during synthesis.
Table 2: Magnetic Properties of Core-Shell Nanoparticle Systems
| Core-Shell Structure | Key Magnetic Properties | Effect of Structure on Properties |
|---|---|---|
| ZnFe₂O₄ @ NiFe₂O₄ [66] | Higher saturation magnetization; Doubled blocking temperature; Improved spin coupling. | Coherent interface and improved crystallinity from the shell enhance magnetic coherence and stability. |
| Co-oxide / Co / Co-oxide (Core/Shell/Shell) [65] | Coercivity increased by 3 orders of magnitude; Exchange bias strength increased by 4 orders of magnitude. | Extra exchange coupling at the shell-shell interface dramatically enhances coercivity and bias. |
| Co-oxide / Co (Inverted Core/Shell) [65] | Lower coercivity and exchange bias vs. core/shell/shell. | Single interface provides less exchange coupling compared to dual-interface structures. |
| Fe₃O₄ @ TiO₂ [64] | Superparamagnetism; Lower saturation magnetization than pure Fe₃O₄. | The non-magnetic TiO₂ shell dilates the magnetic signal, reducing saturation magnetization. |
The Role of Crystallinity and Structural Control
Crystallinity—the degree of structural order in a crystalline material—profoundly impacts magnetic properties. Enhanced crystallinity typically reduces defects that can pin magnetic domains, leading to improved magnetic saturation and more predictable behavior.
Crystallinity and Magnetic Performance
The synthesis of a NiFe₂O₄ shell around a ZnFe₂O₄ core was found to improve the net crystallinity of the overall composite material [66]. This improvement in crystallinity, coupled with a reduction in surface convexity, contributed to more effective spin coupling at the interface. The combined effect of these structural and morphological changes resulted in the observed enhancement of saturation magnetization and blocking temperature [66]. This demonstrates that crystallinity control is not merely a secondary factor but an integral part of optimizing magnetic performance in nanostructured systems.
Furthermore, the general structural and chemical aspects of perovskites, such as crystal symmetry and the presence of structural distortions (e.g., tilting of BO₆ octahedra), are known to strongly influence key magnetic parameters [11]. Advances in synthesis techniques have enabled precise control over defect management and nanostructuring, which are critical for enhancing magnetic performance [11].
Experimental Protocols and Methodologies
Reproducible synthesis and thorough characterization are fundamental to the development of advanced magnetic materials. Below are detailed protocols for key methodologies cited in this field.
Objective: To synthesize rutile-phase TiO₂ nanoparticles doped with magnetic ions (e.g., Fe, rare-earth elements). Materials: Titanium precursor (e.g., titanium isopropoxide), dopant precursors (e.g., Fericip XT, cerium nitrate, erbium nitrate, neodymium nitrate), solvent (e.g., ethanol), deionized water, catalyst (e.g., nitric acid). Procedure:
- Precursor Solution Preparation: Dissolve the titanium precursor in a suitable solvent (e.g., ethanol) under vigorous stirring.
- Dopant Addition: Add the calculated amount of dopant precursor solution to the titanium precursor solution to achieve the desired doping concentration (e.g., 1%, 3%, 5%).
- Hydrolysis and Gelation: Slowly add a mixture of deionized water and a catalyst to the solution under continuous stirring to initiate hydrolysis. This will lead to the formation of a wet gel.
- Aging: Allow the gel to age at room temperature for several hours (e.g., 12-24 hours) to complete the reaction and stabilize the network.
- Drying: Dry the gel in an oven at a moderate temperature (e.g., 60-80°C) to remove the solvent and obtain a xerogel.
- Calcination: Anneal the dried powder in a furnace at elevated temperatures (e.g., 400-600°C) for several hours to crystallize the nanoparticles into the desired phase (rutile, in this case).
Objective: To fabricate bi-magnetic core-shell nanoparticles, specifically ZnFe₂O₄@NiFe₂O₄. Materials: Core nanoparticles (e.g., pre-synthesized ZnFe₂O₄ nanospheres), shell precursors (e.g., nickel nitrate and iron nitrate), solvent, pH regulator (e.g., sodium hydroxide). Procedure:
- Core Synthesis: Pre-synthesize monodisperse core nanoparticles (e.g., ZnFe₂O₄) using a method such as co-precipitation or thermal decomposition.
- Seed Dispersion: Disperse the core nanoparticles uniformly in a solvent using ultrasonication.
- Shell Precursor Addition: Slowly add the shell metal precursor solutions to the core dispersion under controlled stirring.
- Controlled Shell Growth: Carefully adjust the reaction conditions, such as temperature and pH, to facilitate the heterogeneous nucleation of the shell material (NiFe₂O₄) onto the core seeds rather than homogeneous nucleation in the solution.
- Heating and Stirring: Maintain the reaction mixture at an elevated temperature with continuous stirring for a specific duration to allow for the complete growth of the shell to the desired thickness.
- Purification: Isolate the core-shell nanoparticles by centrifugation and wash repeatedly with solvent and/or deionized water to remove unreacted precursors and by-products.
- Drying: Dry the purified nanoparticles in an oven to obtain the final powder.
Objective: To prepare singly inverted (Co-oxide/Co) and core/shell/shell (Co-oxide/Co/Co-oxide) nanostructures. Materials: Co₂O₃ powder, high-purity hydrogen gas, high-purity oxygen gas, tube furnace. Procedure:
- Synthesis of AFM Core: Reduce Co₂O₃ powder in a pure hydrogen atmosphere within a tube furnace at a temperature between 180°C and 500°C for approximately 1 hour to produce an antiferromagnetic Co-oxide (CoO) core.
- Inverted Core/Shell Synthesis: Reduce the resulting CoO nanoparticles in a hydrogen atmosphere at a specific temperature (e.g., 370°C). The amount of hydrogen is calculated to reduce only a portion (e.g., 80%) of the Co-oxide, creating a metallic Cobalt (Co) shell and resulting in a Co-oxide/Co structure.
- Core/Shell/Shell Synthesis: Oxidize the Co-oxide/Co nanostructures in a pure oxygen atmosphere at a set temperature (e.g., 260°C) for varying time intervals (e.g., 5 to 80 minutes). This step grows an outer Co-oxide shell, resulting in the final Co-oxide/Co/Co-oxide structure. The oxidation time directly controls the thickness of the outer shell.
Characterization Techniques
- X-ray Diffraction (XRD): Used to identify crystal phases, estimate crystallite size using Scherrer's formula, and perform quantitative analysis via the Rietveld method [64] [65].
- Vibrating Sample Magnetometer (VSM): Measures magnetic properties such as saturation magnetization, coercivity, remanence, and exchange bias, often as a function of temperature and applied field [64] [65].
- High-Resolution Transmission Electron Microscopy (HR-TEM): Provides direct imaging of nanoparticle size, morphology, and core-shell structure, confirming the coherence of interfaces [64] [66].
- SQUID Magnetometry: A highly sensitive technique for measuring magnetic properties, especially at low temperatures and for weak magnetic signals [65].
The Scientist's Toolkit: Essential Research Reagents and Materials
The experimental protocols rely on a set of critical reagents and instruments for the synthesis and analysis of advanced magnetic materials.
Table 3: Essential Research Reagents and Materials
| Item Name | Function/Application | Specific Example |
|---|---|---|
| Transition Metal Salts | Precursors for doping and core/shell synthesis. | Nitrates (e.g., Nickel Nitrate, Iron Nitrate), Chlorides, or Acetylacetonates of Fe, Co, Ni. |
| Rare-Earth Metal Salts | Precursors for introducing strong magnetic moments via doping. | Salts of Neodymium (Nd), Cerium (Ce), Gadolinium (Gd), Erbium (Er). |
| Alkoxide Precursors | Metal-organic precursors for sol-gel synthesis of oxide matrices. | Titanium Isopropoxide for TiO₂ synthesis. |
| Core Nanoparticles | Seeds for the growth of core-shell structures. | Pre-synthesized ZnFe₂O₄ or Co-Oxide (CoO) nanoparticles. |
| Reducing/Oxidizing Gases | For controlled reduction and oxidation steps in synthesis. | High-purity Hydrogen (H₂) for reduction; High-purity Oxygen (O₂) for oxidation [65]. |
| Vibrating Sample Magnetometer (VSM) | Measurement of saturation magnetization, coercivity, and remanence. | Used to characterize magnetic properties of synthesized powders or films [64]. |
| SQUID Magnetometer | Highly sensitive measurement of magnetic properties, especially at low temperatures. | Used for detailed studies of exchange bias and blocking temperature [65]. |
| X-ray Diffractometer (XRD) | Determination of crystal structure, phase identification, and crystallite size estimation. | Philips X'Pert Pro diffractometer with Cu Kα radiation [65]. |
The optimization of magnetic properties through doping, core-shell structuring, and crystallinity control represents a sophisticated and highly effective paradigm in materials science. Doping allows for the tailoring of intrinsic magnetic interactions, core-shell architectures exploit interfacial coupling to create novel synergistic effects and enhance stability, and crystallinity control ensures optimal spin transport and magnetic coherence. The experimental protocols for sol-gel synthesis, seed-mediated growth, and reduction-oxidation provide robust pathways for fabricating these advanced materials. As characterization techniques continue to advance, allowing for more precise probing of interfaces and magnetic domains, the potential for designing next-generation magnetic materials for spintronics, data storage, and biomedical applications will expand significantly. The interplay of electrical, magnetic, and thermodynamic properties in these engineered systems will remain a rich area for future research and development.
Addressing Challenges in Scalable Synthesis and High-Throughput Material Discovery
The discovery and development of novel functional materials are fundamental to advancements in sustainable energy, electronics, and healthcare technologies. Traditional materials research methodologies, which investigate single compositions through sequential experimentation, are inherently slow and cannot efficiently navigate the vast, multidimensional search space of potential multinary materials [67]. This perspective discusses the paradigm shift from serendipitous discovery to data-guided exploration of materials, a transition critically dependent on overcoming the significant throughput disparity between modern synthesis and characterization techniques [68]. The core challenge lies in a pronounced bottleneck: high-throughput (HT) synthesis tools can produce materials at rates approaching 10⁴ samples per hour, while conventional characterization methods operate at a sluggish pace of approximately 10¹ samples per hour [68]. This orders-of-magnitude discrepancy severely impedes the discovery pipeline for materials with targeted electrical, magnetic, and thermodynamic behaviors. This whitepaper examines the specific challenges in scalable synthesis and characterization, details experimental protocols and data analysis methods to overcome them, and provides a practical toolkit for researchers aiming to accelerate materials discovery.
Core Challenges and Computational Solutions
The journey from a materials concept to a validated discovery is fraught with several interconnected challenges that stem from the inherent complexity of multinary systems and the limitations of traditional experimental frameworks.
- The Vast Unexplored Compositional Space: The periodic table offers countless combinations of elements, creating an almost infinite search space for new multinary materials. Focusing only on earth-abundant and sustainable elements still leaves millions of possible combinations, particularly in ternary, quaternary, and higher-order systems [67]. Exploring this space with one-composition-at-a-time approaches is simply not feasible.
- The Characterization Bottleneck: As highlighted, a severe throughput imbalance exists. HT synthesis methods, such as inkjet printing and combinatorial sputtering, generate thousands of samples rapidly [67]. However, most characterization techniques are manual, slow, and rigid, often designed for uniform thin films and unable to handle the variable morphologies (e.g., droplet-like samples from ink-based deposition) produced by advanced synthesis tools [68].
- Data Complexity and Management: The combinatorial approach generates multidimensional datasets that correlate composition, structure, processing parameters, and functional properties. Managing, analyzing, and extracting meaningful insights from this data requires robust materials informatics and advanced visualization strategies [67].
To address these challenges, the integration of adaptive computer vision and materials informatics has emerged as a transformative solution. Computer vision algorithms can autonomously identify and analyze arbitrarily many material samples in parallel, regardless of their spatial arrangement or morphology. This parallelization can lead to an 85x faster throughput compared to non-automated workflows, effectively synchronizing characterization rates with synthesis capabilities [68]. Furthermore, computational methods and theoretical predictions are invaluable for down-selecting the most promising regions of the compositional space to explore experimentally, making the discovery process more efficient and less reliant on serendipity [67].
Experimental Methodologies and Workflows
Implementing a high-throughput discovery pipeline requires a cohesive workflow that integrates synthesis, characterization, and data analysis. The following protocols detail the key methodologies.
Combinatorial Synthesis of Materials Libraries
A materials library (ML) is a well-defined set of samples—fabricated in a single experiment—designed for high-throughput characterization [67].
- Protocol for Thin-Film Materials Libraries via Sputtering:
- Library Design: Define the compositional space of interest (e.g., a complete ternary system or a focused region around a predicted composition).
- Deposition: Employ combinatorial magnetron sputtering using multiple targets. Two primary methods are:
- Wedge-Type Multilayer Deposition: Use computer-controlled movable shutters to deposit nanoscale layers oriented at specific angles (e.g., 120° for ternaries). Post-deposition annealing at optimized temperatures induces interdiffusion and phase formation.
- Co-sputtering: Simultaneously co-deposit from multiple sources to create an atomic mixture, often yielding metastable phases when performed at room temperature.
- Processing: Identify appropriate annealing conditions (temperature, atmosphere, duration) to crystallize the as-deposited precursors into desired phases.
Automated High-Throughput Characterization
Closing the throughput gap necessitates automating property measurement. The following protocol outlines a computer vision-driven approach for optical characterization.
- Protocol for Automated Band Gap and Stability Characterization [68]:
- Data Acquisition: Capture a hyperspectral datacube of an entire ML batch, containing spatial and spectral information (R(λ)).
- Sample Detection and Segmentation (Algorithm 1):
- Apply a sequence of edge-detection filters to identify discrete islands (samples) of material.
- Index each sample uniquely based on its position within a graph connectivity network.
- Spatially map the pixel coordinates of each segmented sample to its corresponding reflectance spectrum, generating a segmented datacube, Φ = (X̂, Ŷ, R(λ)).
- Property Calculation:
- Band Gap: autonomously compute the direct band gaps of hundreds of compositions from the reflectance data. The algorithm can process ~200 compositions in about 6 minutes with 98.5% accuracy versus expert manual evaluation.
- Environmental Stability: autonomously quantify degradation from optical data over time, processing ~200 compositions in 20 minutes with 96.9% accuracy.
Workflow Visualization
The entire high-throughput process, from synthesis to analysis, can be visualized in the following workflow diagram.
Diagram 1: High-Throughput Material Discovery Workflow.
Data Analysis and Visualization Strategies
The raw data generated from HT characterization is multidimensional, linking composition, structure, and functional properties. Effective analysis and visualization are critical for extracting insights.
Quantitative Analysis of Composition-Property Relationships
A primary goal is to establish quantitative relationships between the composition of a material and its functional properties. The following table summarizes key electrical, magnetic, and thermodynamic properties that are often mapped.
Table 1: Key Material Properties for High-Throughput Screening
| Property Category | Specific Property | Description & Relevance | Typical Measurement Method |
|---|---|---|---|
| Electrical | Resistivity (ρ) | Measures opposition to electrical current flow; fundamental for conductors/insulators. | 4-point probe [69] |
| Band Gap (E_g) | Energy gap between valence and conduction bands; critical for semiconductors. | UV-Vis Spectroscopy, Hyperspectral Imaging [68] | |
| Magnetic | Saturation Magnetization (M_s) | Maximum intrinsic magnetic moment per unit volume; key for ferromagnetic materials. | Vibrating Sample Magnetometry (VSM) [44] |
| Coercivity (H_c) | Resistance to demagnetization; an extrinsic property important for permanent magnets. | VSM [67] | |
| Thermodynamic | Seebeck Coefficient (S) | Voltage generated per unit temperature difference; defines thermoelectric efficiency. | Seebeck Effect Measurement [70] |
| Thermal Conductivity (κ) | Rate of heat transfer through a material; must be low for thermoelectrics. | Laser Flash Analysis [70] | |
| General Optical | Reflectance / Transmittance | Proportion of light reflected/transmitted by a material; used to derive band gap and stability. | Spectrophotometry, Hyperspectral Imaging [68] |
Data Visualization for Quantitative Analysis
Transforming numerical data into visual representations is essential for identifying trends and patterns. The choice of visualization depends on the story the data tells [71].
- Line Charts: Ideal for displaying the change of a property (e.g., Seebeck coefficient) across a continuous composition gradient (x in FA₁₋ₓMAₓPbI₃) or over time (for degradation studies) [71].
- Stacked Bar Charts: Useful for comparing the breakdown of categorical data, such as the distribution of magnetic phases (ferromagnetic, paramagnetic) across different composition regions [72].
- Scatter Plots: Excellent for revealing correlations or relationships between two quantitative variables, like the electrical conductivity versus thermal conductivity in thermoelectric materials [71].
The Scientist's Toolkit: Essential Research Reagents and Materials
Building a high-throughput materials discovery pipeline requires specific reagents, tools, and software. The following table details key components.
Table 2: Essential Research Reagents and Tools for High-Throughput Experimentation
| Category / Item | Function / Description | Example in Use |
|---|---|---|
| Precursor Materials | Liquid or solid sources of chemical elements for synthesis. | Formamidinium lead iodide (FAPbI₃) and Methylammonium lead iodide (MAPbI₃) precursors for perovskite semiconductor libraries [68]. |
| Combinatorial Sputtering System | Fabricates thin-film materials libraries with composition spreads via co-sputtering or wedge-type multilayer deposition. | Used to explore complete ternary material systems or focused regions around predicted compositions [67]. |
| Hyperspectral Imaging System | Captures spatial and spectral data (reflectance/transmittance) across a wide range of wavelengths for an entire library. | Enables parallelized, non-contact measurement of optical properties used to compute band gap and degradation [68]. |
| Computer Vision Software | Custom algorithms for automated image segmentation, sample identification, and data mapping. | Algorithm 1 for segmenting N=201 unique FA₁₋ₓMAₓPbI₃ samples and mapping them to their reflectance spectra R(λ) [68]. |
| Data Analysis & Visualization Tools | Software for statistical analysis, materials informatics, and creating charts/graphs. | Python (Pandas, NumPy) for handling large datasets; ChartExpo or Powerdrill AI for generating visualizations without coding [72] [71]. |
The challenges in scalable synthesis and high-throughput material discovery are being decisively addressed through the integration of combinatorial materials science, automated characterization driven by computer vision, and sophisticated data analysis. By implementing the experimental protocols and data strategies outlined in this guide, researchers can systematically navigate the vast search space for new materials. This transition from serendipity to a data-driven, accelerated workflow is pivotal for the rapid development of next-generation materials with optimized electrical, magnetic, and thermodynamic behaviors, ultimately powering future technological innovations.
Ensuring Colloidal Stability and Controlled Biodistribution through Surface Engineering
In the realm of advanced materials research, the electrical, magnetic, and thermodynamic behaviors of nanoparticles are fundamental properties that dictate their performance in biomedical applications. Surface engineering has emerged as a critical discipline for modulating these intrinsic properties to achieve two paramount objectives in nanomedicine: ensuring colloidal stability and directing controlled biodistribution. The surface characteristics of a nanoparticle—including its charge, hydrophobicity, and functional groups—serve as the primary interface with biological systems, directly influencing its stability in physiological environments, its journey through the body, and its final destination within tissues and cells [73]. By applying tailored surface modifications, researchers can transform nanoparticles from simple carriers into sophisticated, intelligent systems capable of navigating biological barriers, resisting immune clearance, and delivering therapeutic agents with precision to target sites, thereby aligning their colloidal and biological behaviors with therapeutic goals.
Fundamental Principles of Surface-Property Relationships
The behavior of nanoparticles in a biological milieu is governed by a set of fundamental physicochemical properties determined by their surface characteristics. Understanding these relationships is essential for rational design.
- Surface Charge and Cellular Uptake: The surface charge, or zeta potential, of a nanoparticle dictates its initial interactions with negatively charged cell membranes. Positively charged nanoparticles often demonstrate enhanced cellular uptake due to electrostatic attraction. However, this same property can lead to increased protein adsorption (opsonization) and rapid clearance by the mononuclear phagocyte system (MPS), shortening circulation time [73].
- Hydrophobicity and Stability: The degree of hydrophobicity influences a nanoparticle's tendency to aggregate in aqueous biological fluids. Hydrophobic surfaces can trigger particle aggregation to minimize contact with water, compromising colloidal stability and leading to unpredictable biodistribution. Conversely, hydrophobic surfaces are highly effective for encapsulating poorly water-soluble drugs [73].
- Particle Size and Tissue Penetration: The size of the nanoparticle, a parameter directly influenced by surface coatings, is a critical factor for biodistribution. Nanoparticles in the 10-100 nm range typically exhibit prolonged circulation and enhanced tissue penetration. Particles smaller than 10 nm may undergo rapid renal clearance, while larger particles are more susceptible to MPS sequestration in the liver and spleen [73].
Table 1: Impact of Key Surface Properties on Nanoparticle Behavior
| Surface Property | Impact on Colloidal Stability | Impact on Biodistribution |
|---|---|---|
| Charge (Positive) | May induce aggregation; high protein adsorption | Rapid MPS clearance; enhanced uptake in target cells |
| Charge (Neutral/Negative) | Improved stability; reduced protein adsorption | Prolonged circulation; reduced non-specific uptake |
| Hydrophobicity | High aggregation in biological fluids | Increased MPS clearance; potential for off-target toxicity |
| Hydrophilicity | Enhanced dispersion and stability in blood | Longer circulation half-life; improved bioavailability |
| Particle Size (<10 nm) | High stability but challenging to functionalize | Rapid renal clearance; reduced tumor accumulation |
| Particle Size (10-100 nm) | Good stability with proper surface coating | Optimal for enhanced permeability and retention (EPR) effect |
Surface Engineering Strategies and Their Mechanisms
Surface engineering employs various chemical and biological strategies to coat or functionalize nanoparticle surfaces, directly altering their electrical, magnetic, and thermodynamic interactions with the environment to achieve desired stability and targeting outcomes.
Stealth Coating for Enhanced Stability
The primary goal of stealth coating is to create a "shield" around the nanoparticle that minimizes opsonization and MPS recognition.
- Polyethylene Glycol (PEG): PEGylation is the most established stealth technique. By creating a hydrophilic and steric barrier, PEG chains reduce protein adsorption and phagocytic uptake. The density and chain length of PEG are critical; for instance, a 150% PEGylation density on SN38 prodrug nanoparticles was found to optimally balance stability, circulation time, and tumor accumulation, while lower densities (e.g., 20%) led to poor stability and rapid clearance [74].
- Alternative Polymers: Beyond PEG, other hydrophilic polymers like chitosan can be used. Chitosan imparts a positive charge, which can enhance mucoadhesion and extend residence time at absorption sites, though it may also increase MPS interaction without careful optimization [73].
Active Targeting for Controlled Biodistribution
Active targeting involves conjugating specific ligands to the nanoparticle surface to promote receptor-mediated uptake in target cells.
- Ligand Types: Common targeting moieties include antibodies (for antigen recognition), peptides (e.g., for targeting integrins), and small molecules (e.g., folic acid for folate receptor-overexpressing cancers) [73].
- Engineering Exosomes: The natural targeting properties of exosomes can be harnessed and enhanced through surface engineering. Techniques include genetic engineering of parent cells to express targeting peptides (e.g., rabies viral glycoprotein peptide for brain targeting) or direct chemical modification of the exosomal surface post-isolation [75].
Bio-responsive Surfaces for Stimuli-Responsive Release
Surface coatings can be designed to be "smart," responding to specific environmental stimuli at the target site to trigger drug release.
- pH-Sensitive Polymers: Polymers such as poly(acrylic acid) (PAA) undergo conformational changes in the acidic tumor microenvironment (pH ~6.5-6.8) or within endosomes/lysosomes (pH ~5.0-5.5), leading to the disintegration of the nanoparticle or the removal of a surface gatekeeper to release the drug [76].
- Enzyme-Responsive Linkers: Surfaces can be functionalized with linkers that are cleaved by disease-specific enzymes (e.g., matrix metalloproteinases in tumors), enabling site-specific drug release [76].
Experimental Protocols for Surface Engineering and Evaluation
Robust experimental methodologies are essential for developing and characterizing surface-engineered nanoparticles.
Protocol for PEGylation and Characterization
This protocol outlines the process for creating PEGylated nanoparticles with controlled density, as used in studies of SN38 prodrug nano-assemblies [74].
- Synthesis and PEGylation: SN38 prodrug nanoparticles (NPs) are formed via nano-precipitation. Different molar ratios of the prodrug (Wprodrug) and PEG-lipid (WDSPE-mPEG2k), ranging from 0% to 200%, are combined to create NPs with varying PEG densities (e.g., 0%, 5%, 20%, 40%, 60%, 80%, 100%, 150%, and 200% NPs).
- Colloidal Stability Assessment: The stability of the PEGylated NPs is evaluated by monitoring particle size and polydispersity index (PDI) over time in physiologically relevant buffers (e.g., PBS) and in the presence of serum using dynamic light scattering (DLS). Low PEGylation (e.g., 20%) often results in particle aggregation, while high PEGylation (e.g., 150%) maintains monodisperse populations.
- In Vitro Characterization:
- Drug Release: The release kinetics of SN38 from the NPs are quantified using High-Performance Liquid Chromatography (HPLC) under sink conditions.
- Cellular Uptake: The internalization of NPs into target cells (e.g., CT26 colon cancer cells) is accurately quantified using Liquid Chromatography-Mass Spectrometry (LC-MS) to measure intracellular SN38 levels.
- Cytotoxicity: The therapeutic efficacy of the NPs is assessed via the MTT assay, which measures cell viability after treatment.
Protocol for Evaluating Biodistribution and Pharmacokinetics
This protocol describes how to track the in vivo fate of systemically administered, surface-engineered nanoparticles [74] [75].
- Fluorescent Labeling: Nanoparticles or exosomes are labeled with a lipophilic near-infrared (NIR) fluorescent dye (e.g., DiR or DID) that incorporates into the lipid bilayer.
- Animal Models: The labeled particles are administered intravenously to animal models, such as Sprague-Dawley rats for pharmacokinetic studies or CT26 tumor-bearing BALB/c mice for biodistribution and efficacy studies.
- In Vivo Imaging: At predetermined time points, whole-body fluorescence imaging is performed using an in vivo imaging system (IVIS) to non-invasively track the real-time distribution of the nanoparticles.
- Ex Vivo Analysis: At the endpoint, animals are euthanized, and major organs (liver, spleen, kidney, lung, heart, tumor) are harvested. The fluorescence intensity in each organ is measured to quantitatively determine the percentage of injected dose per gram of tissue (%ID/g). This confirms that highly PEGylated NPs (150%) achieve superior tumor accumulation compared to low-PEGylated or non-PEGylated counterparts.
The following workflow summarizes the key stages of surface engineering and evaluation:
Essential Research Reagent Solutions
The following table catalogues critical reagents and materials used in the surface engineering and evaluation of nanoparticles for drug delivery.
Table 2: Key Research Reagents for Surface Engineering Studies
| Reagent / Material | Function in Research | Specific Example from Literature |
|---|---|---|
| DSPE-mPEG₂₀₀₀ | A PEG-lipid conjugate used for creating stealth coatings on nanoparticles, reducing opsonization and prolonging circulation. | Used to create SN38 prodrug NPs with PEGylation levels from 0% to 200% to study its impact on pharmacokinetics [74]. |
| Near-Infrared (NIR) Dyes (DiR, DiD) | Lipophilic fluorescent dyes for labeling the lipid bilayer of nanoparticles or exosomes to enable tracking of biodistribution using in vivo imaging systems (IVIS). | Used to monitor the real-time distribution and organ accumulation of systemically administered exosomes and nanoparticles [75]. |
| Targeting Ligands (Peptides, Antibodies) | Molecules conjugated to the nanoparticle surface to facilitate active targeting to specific cell receptors, improving site-specific delivery. | Genetically engineered onto exosomes (e.g., RVG peptide for brain targeting) or chemically conjugated to polymeric NPs [75]. |
| pH-Sensitive Polymers (e.g., PAA) | Used to create bio-responsive surfaces that undergo structural change in the acidic tumor microenvironment, triggering drug release. | Formulated into nanogels that release chemotherapeutic agents like SN38 specifically at the tumor site [76]. |
| Mesoporous Silica Nanoparticles (MSNs) | Inorganic nanoparticles with high surface area and tunable pores, serving as a versatile platform for surface functionalization and drug loading. | Used as drug delivery agents; surface can be functionalized with polymers and targeting ligands for controlled release [77]. |
Quantitative Data and Case Studies
Empirical data is crucial for understanding the quantitative impact of surface engineering. A pivotal study on SN38 prodrug nanoparticles provides a clear correlation between PEGylation density and biological performance.
Table 3: Impact of PEGylation Density on SN38 Prodrug Nanoparticle Performance [74]
| PEGylation Level | Colloidal Stability | Cellular Uptake (In Vitro) | Circulation Half-Life (In Vivo) | Tumor Accumulation | Antitumor Efficacy |
|---|---|---|---|---|---|
| Low (20%) | Poor (aggregation) | Reduced | Short (rapid MPS clearance) | Low | Minimal |
| Moderate (80%) | Improved | High | Moderate | Moderate | Moderate |
| High (150%) | Excellent | Slightly Reduced | Long (prolonged) | High | Superior |
| Very High (200%) | Excellent | Further Reduced | Long | High (similar to 150%) | High (but risk of ABC effect) |
The data demonstrates a critical trade-off: while high PEGylation is essential for in vivo stability and pharmacokinetics, it can slightly reduce cellular uptake in vitro. This underscores the necessity of optimizing surface engineering for the intended biological environment, as in vitro performance does not always predict in vivo success.
The relationship between surface properties and the resulting biological fate can be visualized as a flow of decisions and outcomes:
Surface engineering represents a powerful and indispensable tool for bridging the gap between the innate electrical, magnetic, and thermodynamic properties of nanomaterials and the complex demands of biological systems. By meticulously designing surface characteristics such as charge, hydrophilicity, and ligand presentation, researchers can exert precise control over two of the most critical challenges in nanomedicine: colloidal stability and biodistribution. The strategies outlined—from stealth coating with PEG to active targeting with specific ligands—provide a robust framework for developing next-generation nanotherapeutics. As the field progresses, the integration of bio-responsive elements and advanced computational modeling will further refine our ability to engineer surfaces that dynamically interact with the disease microenvironment. This relentless pursuit of precision in surface design is fundamental to realizing the full potential of nanotechnology in achieving targeted, effective, and safe therapeutic outcomes.
Integrating Computational Design with Experimental Validation for Accelerated Development
The paradigm of materials research, particularly in the study of electrical, magnetic, and thermodynamic behaviors, is undergoing a profound transformation. The traditional Edisonian approach, reliant on intuition and serendipity, is increasingly being supplemented by a rigorous, collaborative methodology that integrates advanced computation with experimental validation [78]. This integration is creating an accelerated pathway for the design and development of novel materials. The core of this methodology lies in using computational tools not merely for rationalizing experimental results but for actively predicting and designing new materials with targeted properties, which are then synthesized and tested in the laboratory [79]. This closed-loop feedback between in-silico prediction and experimental reality is essential for building robust, reliable models and for achieving a genuine acceleration of the development cycle, a principle that underpins initiatives like the Materials Genome Initiative [80].
Core Methodology: A Cyclical Workflow
The integration of computational design and experimental validation is not a linear process but a cyclical, iterative workflow. Each cycle refines the computational models and deepens the physical understanding of the material system. A generalized, effective workflow can be broken down into four key stages, as visualized below.
Stage 1: High-Throughput Computational Screening & Design
The process begins with the computational generation of a large library of candidate materials. Density Functional Theory (DFT) is a cornerstone of this stage, providing insights into fundamental electronic, magnetic, and thermodynamic properties [80]. For larger systems, such as Multi-Principal Element Alloys (MPEAs), classical Molecular Dynamics (MD) simulations are employed, though their accuracy is contingent on the quality of the interatomic potentials [81]. The scale of this exploration is enabled by high-throughput computing frameworks, which have led to the creation of extensive databases like the Materials Project, AFLOWLIB, and JARVIS-DFT [78] [80]. These databases host calculated properties for tens of thousands of materials, serving as a primary source for initial screening.
To navigate this vast compositional and structural space efficiently, Machine Learning (ML) and Deep Learning (DL) have become indispensable. These models act as surrogate models, rapidly predicting material properties at a fraction of the computational cost of full-scale simulations [81]. The trend is moving beyond using these models as "black boxes" towards Explainable AI (XAI). Techniques like SHapley Additive exPlanations (SHAP) analysis are used to uncover the physical and chemical drivers behind the model's predictions, such as revealing the relationship between elemental concentration or local chemical order and a target property like unstable stacking fault energy [81].
Stage 2: Candidate Selection & Experimental Synthesis
Following computational screening, the most promising candidates are selected for experimental realization. This requires close collaboration between theoreticians and experimentalists to ensure that the synthesized materials correspond to the computed models. For the study of MPEAs, this typically involves arc-melting or induction melting under an inert atmosphere to produce homogeneous, bulk alloy buttons from high-purity elemental constituents [81]. These buttons are often subsequently homogenized through high-temperature annealing to achieve a equilibrium single-phase state, a critical step for validating predictions of phase stability [81]. For other material classes, such as thin films for electronic applications, combinatorial synthesis techniques like sputtering or pulsed laser deposition can be used to rapidly create libraries of compositions on a single substrate, allowing for efficient experimental mapping of phase diagrams and functional properties [80].
Stage 3: Advanced Characterization & Property Validation
Once synthesized, the materials undergo rigorous characterization to validate the computational predictions. The core methodology involves:
- Structural and Phase Analysis: X-ray Diffraction (XRD) is used to confirm the crystal structure (e.g., face-centered cubic, body-centered cubic) and to verify the predicted single-phase nature of the material [81].
- Microstructural and Chemical Analysis: Scanning Electron Microscopy (SEM) combined with Energy-Dispersive X-ray Spectroscopy (EDS) is employed to examine microstructure, assess homogeneity, and verify that the chemical composition matches the intended design [81].
- Functional Property Measurement: This is the direct test of the computational predictions. For mechanical properties, nanoindentation is a common technique to measure hardness and Young's modulus [81]. For electronic and magnetic properties, techniques such as four-point probe measurements for electrical conductivity and Vibrating Sample Magnetometry (VSM) for magnetic hysteresis are standard. Thermodynamic properties may be measured via differential scanning calorimetry (DSC).
Stage 4: Data Integration & Model Refinement
The final, crucial stage is the integration of experimental results back into the computational workflow. Discrepancies between predicted and measured properties are not failures but valuable sources of information. These discrepancies can be used to refine the underlying models, whether by improving the accuracy of DFT functionals, optimizing ML feature sets, or retraining ML/DL models on the newly acquired experimental data [79] [80]. This feedback loop, potentially guided by uncertainty quantification, progressively improves the predictive power of the computational tools, making each successive design cycle more efficient and accurate. This creates a powerful, self-improving research ecosystem where knowledge is continuously aggregated and leveraged [80].
The following tables consolidate key quantitative data from successful applications of this integrated approach, highlighting the predictive capabilities of computation and the role of experimental validation.
Table 1: Validated Material Properties from an Inverse-Designed FeNiCrCoCu Multi-Principal Element Alloy (MPEA) Study [81]
| Composition / Property | Computational Prediction (MD) | Experimental Measurement | Validation Outcome |
|---|---|---|---|
| FeNiCrCoCu (Variant 1) | High USFE, Single-Phase FCC | Single-Phase FCC (XRD) | Qualitative Agreement: Phase stability correctly predicted. |
| Young's Modulus | Predicted high value | High value (Nanoindentation) | Qualitative Agreement: Trend in mechanical properties confirmed. |
| Key XAI Insight | SHAP analysis revealed local Co/Cr clustering and its correlation with USFE and modulus. | N/A | Provides a physical, atomistic rationale for the design. |
Table 2: Prominent Computational Materials Databases and Their Scope [78] [80]
| Database Name | Primary Focus | Scale of Data | Example Application in Design |
|---|---|---|---|
| Materials Project | DFT-calculated properties of inorganic compounds | 100,000+ materials | Screening for Li-ion battery electrodes, photovoltaic materials. |
| AFLOWLIB | High-throughput DFT calculations | Millions of calculated compounds | Crystal structure prototype analysis, stability predictions. |
| JARVIS-DFT | DFT, FF, and ML for diverse materials | 30,000+ materials | Discovery of 2D materials, topological insulators, and exfoliation energies. |
| C2DB | Properties of 2D materials | 3,500+ 2D materials | Design of Janus structures, 2D semiconductors, and topological materials. |
| OQMD | DFT-based thermodynamic and electronic properties | 1,000,000+ calculated structures | High-throughput phase stability assessment. |
Detailed Experimental Protocol: Synthesis and Validation of an Inverse-Designed MPEA
This protocol provides a detailed methodology for the experimental validation of a computationally designed MPEA, as referenced in Section 3.
Synthesis via Arc-Melting
Objective: To produce a bulk, homogeneous specimen of the target composition (e.g., a specific FeNiCrCoCu variant) from pure elemental components.
- Materials:
- High-purity elemental chunks/wires (>99.9% pure) of Fe, Ni, Cr, Co, and Cu.
- Titanium getter: for oxygen gettering during melting.
- Water-cooled copper hearth: as the crucible for melting.
- Procedure:
- Weighing: Precisely weigh the individual elements according to the atomic percentages defined by the computational prediction.
- Environment Purge: Place the constituent elements on the copper hearth inside the arc-melter chamber. Evacuate the chamber to a high vacuum (e.g., <10⁻⁵ mbar) and backfill with high-purity argon gas. Repeat this purge cycle 2-3 times to minimize oxygen contamination.
- Melting: Melt the Ti getter first to further scavenge any residual oxygen. Then, apply the arc to the mixture of constituent elements. To ensure homogeneity, flip and re-melt the alloy button at least five times.
- Cooling: After the final melt, allow the button to cool inside the chamber under an argon atmosphere.
Homogenization Annealing
Objective: To promote interdiffusion of atoms and achieve a chemically homogenous, equilibrium single-phase state.
- Procedure:
- Seal the as-cast alloy button in an evacuated quartz ampoule backfilled with a partial pressure of argon to prevent oxidation.
- Heat the ampoule in a box furnace at a high temperature (e.g., 1100-1300°C) for a prolonged period (e.g., 48 hours) to facilitate atomic diffusion.
- After the annealing step, quench the ampoule in water to retain the high-temperature phase.
Structural and Mechanical Characterization
Objective: To validate the predicted crystal structure and mechanical properties.
- X-ray Diffraction (XRD):
- Equipment: X-ray diffractometer with Cu Kα radiation.
- Protocol: Mount a flat, polished section of the annealed sample. Perform a scan, for example, from 20° to 100° (2θ). Identify the resulting diffraction peaks and compare them to known reference patterns for FCC, BCC, or other phases to confirm the predicted single-phase nature.
- Nanoindentation:
- Equipment: Nanoindenter with a Berkovich diamond tip.
- Protocol: Prepare a metallographically polished sample surface. Perform a grid of indents (e.g., 5x5) at specified load (e.g., 50 mN). Use the Oliver-Pharr method to analyze the load-displacement curves from these indents to extract the reduced modulus and hardness. Report the average and standard deviation of these measurements.
The Scientist's Toolkit: Essential Research Reagents & Materials
This table details key resources used in the computational and experimental workflows described in this guide.
Table 3: Key Research Reagents and Computational Tools for Integrated Materials Development
| Item Name | Function / Application | Specific Examples / Notes |
|---|---|---|
| High-Purity Elements | Starting materials for synthesis of bulk alloys via melting. | Fe, Ni, Cr, Co, Cu chunks/wires (>99.9% purity) to minimize impurity effects [81]. |
| DFT Software | First-principles calculation of electronic structure, magnetic moments, and phase stability. | VASP, Quantum ESPRESSO (used by databases like Materials Project and AFLOWLIB) [80]. |
| Molecular Dynamics Code | Simulating thermodynamic and mechanical properties of larger systems (1000+ atoms). | LAMMPS, GROMACS; requires carefully chosen interatomic potentials [81]. |
| Machine Learning Libraries | Building surrogate models for rapid property prediction and inverse design. | scikit-learn (for ML models), TensorFlow/PyTorch (for DL models) [80]. |
| SHAP Library | Explainable AI tool for interpreting ML/DL model predictions and gaining physical insights. | Identifies key compositional or structural features driving properties like USFE [81]. |
| Characterization Suite | Validating the structure, composition, and properties of synthesized materials. | XRD (structure), SEM/EDS (microstructure/composition), Nanoindenter (mechanical properties) [81]. |
Visualizing the Multi-Scale Data Integration Framework
The power of the integrated approach is fully realized when data from multiple sources and length scales are combined within a unified framework. The following diagram illustrates this holistic vision, which connects high-throughput computation, macroscopic experiments, and atomic-scale imaging through data-driven models and statistical physics.
Performance Benchmarking and Preclinical Validation Frameworks
Comparative Analysis of Magnetic Saturation and Specific Absorption Rates Across Material Classes
In the evolving landscape of materials science, the interplay between magnetic saturation (M_s) and Specific Absorption Rate (SAR) represents a critical frontier for advancing technologies in biomedicine, energy, and computing. SAR quantifies the energy absorption rate of electromagnetic fields by materials, defined as the power absorbed per unit mass, with units of watts per kilogram (W/kg) [82]. Magnetic saturation denotes the maximum magnetic moment per unit volume a material can achieve under an applied magnetic field [83] [84]. The relationship between these parameters is foundational for designing functional materials where controlled energy absorption and magnetic responsiveness are paramount, such as in magnetic hyperthermia for cancer therapy, spintronic devices, and energy-efficient computing systems [12] [85] [11].
This analysis examines the magnetic saturation and SAR characteristics across diverse material classes, including spinel ferrites, perovskite oxides, and emerging quantum materials. The focus is on their performance under alternating magnetic fields, the underlying thermodynamic mechanisms, and the implications for current and future technological applications. Understanding the quantitative dependence of SAR on magnetic field parameters and intrinsic material properties provides a framework for tailoring materials to specific operational requirements across interdisciplinary fields.
Theoretical Foundations: SAR and Magnetic Saturation
Defining Specific Absorption Rate (SAR)
The Specific Absorption Rate (SAR) is a critical parameter characterizing the energy conversion efficiency of a material exposed to an alternating electromagnetic field. It is formally defined as the time derivative of the incremental energy (dW) absorbed by an incremental mass (dM) of a material [82]:
[ SAR = \frac{d}{dt} \left( \frac{dW}{dM}\right) = \frac{d}{dt} \left( \frac{dW}{\rho dV}\right) \quad [\text{W/kg}] ]
where ( \rho ) represents the mass density of the material. In practical applications, SAR is often calculated by measuring the induced electric field ( E ) within the material using the relationship [82]:
[ SAR = \frac{\sigma |E|^2}{\rho} ]
where ( \sigma ) denotes the electrical conductivity. For magnetic nanoparticles used in therapeutic applications like magnetic fluid hyperthermia, SAR is experimentally determined through calorimetric measurements by tracking temperature rise over time [85]:
[ SAR = C \frac{ms}{mM} \frac{dT}{dt} ]
where ( C ) is the specific heat capacity of the fluid, ( ms ) is the mass of the magnetic fluid, ( mM ) is the mass of the magnetic element, and ( dT/dt ) is the initial slope of the temperature versus time curve [82].
Magnetic Saturation and Its Role in SAR
Magnetic saturation (Ms) represents the maximum magnetic moment per unit volume that a material can exhibit under an applied magnetic field, beyond which no further increase in magnetization occurs. This intrinsic property is governed by the material's composition, crystal structure, and temperature [83] [11] [84]. Materials with higher Ms values generally demonstrate enhanced SAR values due to greater hysteresis losses and improved energy conversion efficiency under alternating magnetic fields [84].
The relationship between SAR and magnetic field parameters follows a complex dependence. According to the Linear Response Theory (LRT), SAR exhibits a quadratic dependence on the alternating magnetic field amplitude (H) at lower field strengths [83]. However, as field strength increases, saturation effects become significant, causing SAR values to deviate from this quadratic relationship and eventually plateau, forming a sigmoidal dependence on the applied field amplitude [83] [84].
Fundamental Energy Conversion Mechanisms
The transformation of electromagnetic energy into thermal energy in magnetic materials occurs primarily through two distinct physical mechanisms, each dominant under specific conditions:
- Hysteresis Losses: Predominant in multi-domain ferromagnetic or ferrimagnetic materials, this mechanism involves energy dissipation due to domain wall displacement during the magnetization cycle. The area enclosed within the hysteresis loop corresponds directly to the energy converted to heat per cycle [85].
- Relaxation Losses: Dominant in superparamagnetic nanoparticles, relaxation losses occur through two pathways:
- Néel Relaxation: Involves the internal rotation of the magnetic moment within the nanoparticle against its magnetic anisotropy energy barrier, with a characteristic relaxation time dependent on the anisotropy field and temperature [83].
- Brownian Relaxation: Results from the physical rotation of the entire nanoparticle within a viscous medium, governed by the hydrodynamic volume and medium viscosity [83].
The prevailing mechanism in any given system depends on factors including particle size, magnetic anisotropy, and the viscosity of the surrounding medium. In practice, both mechanisms often operate concurrently, with the faster process dominating the overall energy dissipation [83].
Figure 1: SAR mechanisms in magnetic materials under alternating magnetic fields (AMF). Hysteresis losses dominate in multi-domain materials, while relaxation mechanisms prevail in superparamagnetic nanoparticles (NPs), with Néel and Brown relaxation operating in parallel.
Quantitative SAR and Magnetic Saturation Across Material Classes
Spinel Ferrite Nanoparticles
Spinel ferrites represent one of the most extensively studied classes of magnetic materials for applications requiring controlled heat generation, particularly in biomedical contexts. These materials demonstrate a wide range of magnetic properties and SAR values tunable through cation substitution and synthetic control.
Table 1: Magnetic Properties and SAR Values of Spinel Ferrite Nanoparticles
| Material | Size (nm) | Saturation Magnetization (Am²/kg) | Coercivity (kA/m) | SAR Value (W/g) | Field Conditions (kHz, kA/m) |
|---|---|---|---|---|---|
| Zn~0.3~Fe~2.7~O~4~ | 16 | 120 | - | - | Not specified [83] |
| CoFe~2~O~4~ | 12-14 | - | 9-11 | 1780 | 355, 65 [84] |
| MnFe~2~O~4~ | 12-14 | - | 9-11 | 835 | 355, 65 [84] |
| NiFe~2~O~4~ | 12-14 | - | 9-11 | 540 | 355, 65 [84] |
Zinc doping in magnetite (Zn~x~Fe~3-x~O~4~) demonstrates the significant influence of composition on magnetic properties. With increasing zinc content (x value) up to approximately 0.3, saturation magnetization rises substantially to 120 Am²/kg, enhancing potential SAR performance. Beyond this optimal doping level, further zinc incorporation reduces magnetization, illustrating the delicate balance required in compositional engineering [83].
Among spinel ferrites, cobalt ferrite (CoFe~2~O~4~) nanoparticles exhibit the highest SAR values (1780 W/g), followed by manganese ferrite (MnFe~2~O~4~) at 835 W/g, and nickel ferrite (NiFe~2~O~4~) with the lowest heating performance (540 W/g) under identical field conditions (355 kHz, 65 kA/m) [84]. This performance hierarchy correlates directly with the magnetic hardness and saturation magnetization of these materials, with harder magnetic phases generally demonstrating superior heating capabilities at higher field strengths.
The SAR dependence on magnetic field amplitude follows a characteristic sigmoidal profile across all spinel ferrites, deviating from the predicted quadratic relationship of the Linear Response Theory at higher field strengths. This indicates the onset of magnetic saturation effects that limit further enhancement of SAR with increasing field amplitude [84].
Perovskite Materials
Perovskite oxides with the general formula ABO~3~ represent another important class of functional magnetic materials, particularly valuable for their multifunctional properties and tunable magnetic behaviors.
Table 2: Magnetic Properties and Applications of Perovskite Materials
| Material Class | Key Magnetic Properties | Primary Applications | SAR Relevance |
|---|---|---|---|
| Manganites (e.g., La~1-x~Sr~x~MnO~3~) | Colossal Magnetoresistance (CMR) | Magnetic field sensors, spintronics [11] | Indirect through magnetocaloric effect |
| Ferrites | Ferromagnetism, high Curie temperature | Data storage, MRI contrast agents [11] | Direct hyperthermia applications |
| La(Fe, Si)~13~-based compounds | Giant Magnetocaloric Effect (MCE) | Magnetic refrigeration [11] | Alternative thermal management |
Perovskite manganites exhibit remarkable phenomena such as colossal magnetoresistance (CMR), where electrical resistance changes dramatically in response to an applied magnetic field. This effect stems from the complex interplay between electronic structure and magnetic ordering, primarily governed by the double exchange mechanism [11]. While not typically employed for SAR-related applications, the strong coupling between magnetic, electronic, and lattice degrees of freedom in these materials offers potential for novel thermal management applications.
The magnetocaloric effect (MCE) in certain perovskite systems, such as La(Fe, Si)~13~-based compounds, demonstrates an alternative pathway for thermal energy control through magnetic field manipulation. Materials exhibiting large MCE show temperature changes upon application or removal of magnetic fields, providing a foundation for energy-efficient refrigeration technologies [11]. This mechanism represents a complementary approach to thermal management compared to direct SAR-based heating.
The flexibility of the perovskite structure enables extensive property tuning through A-site and B-site cation substitutions, which systematically modify magnetic interactions, Curie temperatures, and overall thermal responses to electromagnetic fields [11]. This tunability makes perovskites particularly valuable for applications requiring specific magnetic and thermal responses under varying operational conditions.
Emerging and Non-Collinear Magnetic Materials
Recent research has uncovered novel magnetic materials with unconventional properties that offer new pathways for controlling SAR and magnetic responses:
- Altermagnets: This newly recognized class of magnetic materials exhibits collinear but compensated magnetic order that defies traditional classification as either ferromagnets or antiferromagnets. These materials may enable unprecedented control over spin-polarized currents with potential applications in ultrafast, low-power computing [86].
- Non-Collinear Spin Textures: Materials with non-collinear magnetic structures, including magnetic skyrmions and spin spirals, demonstrate unusual electrical responses under alternating currents. These responses were initially attributed to emergent inductance effects but may also involve significant Joule heating contributions that influence apparent SAR characteristics [8].
- Magnon-Based Systems: Recent research has revealed that magnons—quantized spin waves propagating through magnetic materials—can generate measurable electric signals in antiferromagnetic materials. This discovery bridges magnetism and electricity without charge flow, potentially enabling computing paradigms with significantly reduced energy dissipation compared to conventional electronics [12].
These emerging material classes expand the conceptual framework for understanding and manipulating SAR effects beyond traditional mechanisms, potentially enabling new applications in quantum information processing, ultra-efficient computing, and advanced thermal management.
Experimental Methodologies for SAR Evaluation
Calorimetric Measurement Techniques
The calorimetric method represents the most direct approach for experimental SAR determination, relying on temperature monitoring during magnetic field application. The standard protocol involves:
Sample Preparation: Magnetic nanoparticles are suspended in a liquid carrier at precisely controlled concentrations, typically ranging from 1-4 mg/mL to ensure uniform heat distribution and prevent aggregation effects [84]. For immobilized particle systems, samples may be aligned in a uniform static magnetic field before solidification to enhance SAR values through preferential orientation [83].
Experimental Setup: The sample is placed within an induction coil or parallel-plate capacitor system capable of generating alternating magnetic fields with controlled amplitude (typically 5-65 kA/m) and frequency (generally 50-1000 kHz) [82] [84]. Temperature monitoring employs fiber-optic thermometers or infrared imaging to avoid interference with electromagnetic fields.
Data Collection: The sample temperature is recorded continuously during application of the alternating magnetic field, with particular attention to the initial linear phase of temperature increase (typically the first 30-60 seconds) [85].
SAR Calculation: The SAR value is calculated from the initial slope of the temperature versus time curve using the formula:
[ SAR = \frac{C}{c_m} \cdot \frac{dT}{dt} ]
where ( C ) represents the total heat capacity of the system, ( c_m ) is the mass concentration of magnetic material, and ( dT/dt ) is the initial temperature rise rate [85].
Figure 2: Standard experimental workflow for SAR determination using calorimetric methods, highlighting critical preparation steps and measurement parameters that influence result accuracy and reproducibility.
Magnetic Characterization Techniques
Complementary magnetic characterization provides essential insights into the fundamental properties governing SAR performance:
Vibrating Sample Magnetometry (VSM): Measures saturation magnetization (Ms), coercivity (Hc), and remanence (M_r) through the DC hysteresis loop, providing crucial parameters for predicting SAR potential [83] [84].
AC Susceptometry: Characterizes the dynamic magnetic response by measuring the complex magnetic susceptibility (χ' + iχ") as a function of frequency, directly probing the relaxation mechanisms responsible for heat generation [83].
Elemental Analysis: Quantifies composition and doping levels in substituted ferrites and perovskites using techniques like energy-dispersive X-ray spectroscopy (EDS) or inductively coupled plasma mass spectrometry (ICP-MS), correlating chemical composition with magnetic properties [83] [11].
Challenges and Standardization in SAR Measurement
Significant experimental challenges complicate SAR evaluation and comparison across different studies:
Field Parameter Limitations: For biomedical applications, safety considerations constrain the applicable field parameters. The Brezovich criterion establishes an upper limit for the product of frequency and field amplitude (f·H ≤ 4.85×10^8 A·m⁻¹·s⁻¹) to avoid non-specific tissue heating, though more permissive limits (f·H ≤ 5×10^9 A·m⁻¹·s⁻¹) have also been proposed [85].
Concentration Dependence: SAR values demonstrate significant concentration dependence due to interparticle interactions and aggregation effects, complicating comparison between studies employing different concentration ranges [84].
Saturation Effects: The observed deviation from quadratic field dependence at higher field amplitudes necessitates reporting complete field dependence profiles rather than single-point measurements for meaningful material comparisons [83] [84].
Instrumentation Variability: Differences in coil design, field calibration, and temperature measurement techniques introduce substantial variability between laboratories, highlighting the need for standardized protocols and reference materials [85].
These challenges underscore the importance of comprehensive reporting of experimental parameters including particle concentration, medium viscosity, field amplitude and frequency, and measurement methodology to enable valid cross-study comparisons.
The Researcher's Toolkit: Essential Materials and Reagents
Table 3: Essential Research Reagents and Materials for SAR and Magnetic Studies
| Reagent/Material | Function | Application Context |
|---|---|---|
| Spinel Ferrite NPs (MFe~2~O~4~, M=Fe, Co, Mn, Ni, Zn) | Primary heating elements | Magnetic hyperthermia, targeted drug delivery [83] [84] |
| Polyol Solvents (e.g., diethylene glycol, tetraethylene glycol) | Synthesis medium and reducing agent | Polyol synthesis of uniform ferrite nanoparticles [84] |
| Surface Ligands (e.g., citric acid, polyacrylic acid, silica coatings) | Surface functionalization, stability, biocompatibility | Colloidal stability, functionalization for targeting [85] |
| Biocompatible Polymers (e.g., chitosan, dextran, phospholipids) | Encapsulation, stealth properties, drug loading | Biomedical applications, in vivo studies [85] |
| Targeting Moieties (e.g., antibodies, peptides, folic acid) | Specific cell targeting | Active targeting in drug delivery systems [85] |
| Alternating Magnetic Field Generators | Application of AC magnetic fields | Experimental SAR measurement, therapeutic applications [82] [85] |
| Reference Materials (e.g., commercial ferrite nanoparticles) | Method validation, interlaboratory comparison | Standardization, quality control [85] |
This toolkit encompasses the essential components required for the synthesis, characterization, and application of magnetic materials in SAR-related research, with particular emphasis on biomedical applications where surface functionalization and biocompatibility are paramount considerations.
Applications and Technological Implications
Biomedical Applications: Magnetic Hyperthermia
Magnetic fluid hyperthermia represents one of the most promising biomedical applications of SAR-controlled materials, utilizing magnetic nanoparticles as localized heat sources for cancer therapy:
Intracellular versus Extracellular Heating: The localization of magnetic nanoparticles significantly influences therapeutic efficacy. While some studies indicate enhanced SAR efficiency for extracellularly positioned nanoparticles due to reduced mobility constraints, in vivo experiments demonstrate superior therapeutic outcomes for intracellularly localized particles, suggesting additional mechanisms beyond bulk heating, including mechanical disruption of cellular structures and localized membrane damage [85].
Combination Therapies: Magnetic hyperthermia functions synergistically with conventional cancer treatments. The localized temperature elevation sensitizes tumor cells to radiation therapy and enhances drug delivery and efficacy in combined hyperthermia-chemotherapy approaches [85].
Targeting Strategies: Both passive and active targeting approaches optimize nanoparticle accumulation in tumor tissues. Passive targeting leverages the enhanced permeability and retention (EPR) effect of tumor vasculature, while active targeting employs surface-bound ligands (antibodies, peptides) recognizing tumor-specific markers [85].
Energy and Computing Applications
The interplay between magnetic saturation and SAR extends beyond biomedical applications into advanced energy and computing technologies:
Spintronic Devices: Perovskite materials with tailored magnetic properties enable novel spintronic devices that exploit both charge and spin degrees of freedom for information processing. The control over magnetization dynamics and spin-polarized currents in these materials facilitates development of memory devices, magnetic sensors, and logic elements with enhanced functionality [11].
Magnon-Based Computing: Recent discoveries demonstrating that magnons can generate measurable electric signals in antiferromagnetic materials open possibilities for computing architectures that merge magnetic and electric systems directly, eliminating energy-wasting conversion steps and enabling operation at terahertz frequencies—approximately a thousand times faster than current magnetic technologies [12].
Magnetic Refrigeration: Perovskite materials exhibiting giant magnetocaloric effects, such as La(Fe, Si)~13~-based compounds, provide the foundation for energy-efficient refrigeration technologies based on controlled magnetic phase transitions rather than gas compression [11].
This comparative analysis reveals the complex interdependence between magnetic saturation and Specific Absorption Rate across diverse material classes, from conventional spinel ferrites to emerging perovskite and quantum materials. The SAR performance of any material derives from the intricate balance between its intrinsic magnetic properties—particularly saturation magnetization and magnetic anisotropy—and extrinsic parameters including alternating magnetic field characteristics and environmental conditions.
Key findings demonstrate that while spinel ferrites offer exceptional SAR values suitable for biomedical hyperthermia applications, perovskite materials provide multifunctional capabilities valuable for spintronics and energy conversion technologies. Emerging material classes including altermagnets and non-collinear spin systems present novel pathways for controlling energy absorption and conversion at the quantum level.
Future research directions should address critical challenges in material design, including the development of standardized SAR measurement protocols, optimization of material properties for specific application environments, and exploration of novel mechanisms for controlling energy conversion at reduced field strengths to enhance biomedical safety. The continued refinement of structure-property relationships in magnetic materials will enable increasingly sophisticated control over SAR characteristics, driving innovations across therapeutics, energy technologies, and information processing.
In Vitro and In Vivo Efficacy Assessment in Neural and Bone Tissue Regeneration Models
The field of regenerative medicine is increasingly leveraging the unique electrical, magnetic, and thermodynamic properties of advanced materials to direct cellular behavior and tissue formation. This technical guide provides a comprehensive framework for assessing the efficacy of novel biomaterials within neural and bone tissue regeneration models, contextualized within the broader study of material behaviors. For researchers and drug development professionals, mastering these assessment protocols is crucial for translating fundamental material science into clinically viable therapies. The intricate interplay between a material's physical properties and the biological environment dictates regenerative outcomes, necessitating robust and standardized evaluation methods from in vitro characterization to in vivo validation. This document details these methodologies, with a specific focus on quantifying regeneration through structural, functional, and biological metrics.
Core Principles: Linking Material Properties to Biological Function
The efficacy of a biomaterial in regeneration is governed by its interaction with the biological system at multiple levels. Key material properties directly influence cellular responses and tissue maturation.
- Electrical Properties: In neural engineering, conductive polymers such as polypyrrole, polythiophene, and polyaniline are employed because they can carry electrical impulses that facilitate neurite outgrowth and enhance neuronal activity by aiding nerve signal travel [87]. For bone, the inherent piezoelectric properties of the native extracellular matrix (ECM) can be mimicked to promote osteogenesis.
- Magnetic Properties: Magnetic materials enable advanced therapeutic strategies such as magnetically-guided cell delivery, remote activation of cellular signaling, and non-invasive monitoring via magnetic resonance imaging (MRI). For instance, MRI techniques have been developed to longitudinally assess neuronal cell death, survival, and regeneration in both animal models and patients, providing a non-invasive method to evaluate treatment efficacy [88].
- Thermodynamic & Structural Properties: Properties like thermal conductivity and heat capacity are critical for material performance and processing [36]. Furthermore, a scaffold's architectural and mechanical properties—including porosity, stiffness, and degradation profile—must mirror the native tissue. A scaffold for the central nervous system should be malleable and structurally sound to integrate without causing inflammation [87].
Neural Tissue Regeneration Models
In Vitro Assessment Protocols
In vitro models are essential for preliminary evaluation of biomaterial biocompatibility and functionality under controlled conditions.
- Primary Cell Cultures: Isolation and culture of primary neurons, Schwann cells, and astrocytes from rodent dorsal root ganglia (DRG) or cortical tissues. These are co-cultured with test scaffolds to assess specific cellular responses [88].
- Stem Cell Cultures: Isolation and osteochondral differentiation of mesenchymal stem cells (MSCs) from bone marrow (BMSCs), umbilical cord (UC-MSCs), or adipose tissue (ASCs). Their low immunogenicity and accessibility make them a suitable cell source [87].
- Cell Line Models: The PC12 cell line (rat pheochromocytoma) is a standard model for neuronal differentiation. Neuronal differentiation is induced by supplementing the culture medium with Nerve Growth Factor (NGF), typically at a concentration of 50-100 ng/mL [89].
Quantitative Morphological Analysis of Neurite Outgrowth:
- Following NGF stimulation, PC12 cells are fixed and stained for neuronal markers such as β-III-tubulin.
- Imaging via fluorescence or confocal microscopy is performed, and neurite length and the number of neurites per cell are quantified using image analysis software (e.g., ImageJ with NeuronJ plugin) [89].
- ELISA for Neurotrophic Factors: Supernatants from scaffold-cell cultures are analyzed using ELISA to quantify the production of key neurotrophins like NGF and Brain-Derived Neurotrophic Factor (BDNF), which are hallmarks of a neuroregenerative environment [89].
In Vivo Assessment Protocols
Translation to in vivo models is critical for evaluating functional integration and repair.
- Peripheral Nerve Injury Model: A sciatic nerve defect model in rats is commonly used. A segment of the nerve is excised, creating a gap (e.g., 10-15 mm) that is bridged with the nerve repair conduit [88].
- Spinal Cord Injury Model: Contusion or transection models in rodents are employed to assess repair strategies for the central nervous system [88].
Efficacy Endpoints and Analytical Methods:
- Histological and Immunohistochemical Analysis: Explanted tissues are sectioned and stained.
- Haematoxylin and Eosin (H&E): For general tissue morphology and cellular infiltration.
- Antibodies against Neurofilament (NF): To visualize regenerating axons.
- Antibodies against Myelin Basic Protein (MBP): To assess remyelination of axons [88].
- Functional Behavior Tests: In spinal cord injury models, the Basso, Beattie, Bresnahan (BBB) locomotor rating scale is used to assess recovery of motor function.
- Advanced Magnetic Resonance Imaging (MRI): Structural and diffusion tensor imaging (DTI) are used non-invasively to assess axonal integrity, track degeneration/regeneration, and visualize neuroplastic changes in the brain following injury [88].
Signaling Pathways in Neural Repair
The following diagram illustrates key molecular mechanisms and signaling pathways involved in neural repair that are targeted by biomaterial strategies.
Bone Tissue Regeneration Models
In Vitro Assessment Protocols
In vitro osteogenic potential is a critical first step in evaluating bone biomaterials.
- Cell Source and Seeding:
- Skeletal Stem Cells (SSCs): Human bone marrow stromal cells (HBMSCs), often enriched for the Stro-1 antigen, are a primary cell source. These are typically encapsulated within the hydrogel scaffold at a density of 1-5 million cells/mL [90].
- Osteogenic Differentiation Culture: Cells are maintained in osteogenic medium, supplemented with 10 mM β-glycerophosphate, 50 µg/mL ascorbic acid, and 100 nM dexamethasone, for up to 28 days, with medium changes twice weekly [90].
Quantitative Analysis of Osteogenesis:
- Alizarin Red S Staining and Quantification: This assay detects calcium-rich deposits. After 21-28 days, cells are fixed and stained with 2% Alizarin Red S (pH 4.2). For quantification, the stain is solubilized with 10% cetylpyridinium chloride, and the absorbance is measured at 562 nm [90].
- Quantitative PCR (qPCR): Expression of osteogenic genes including RUNX2 (early marker), Osteocalcin (OCN), and Osteopontin (OPN) is analyzed. Gene expression is normalized to housekeeping genes like GAPDH and reported as fold-change relative to a control group [91].
In Vivo Assessment Protocols
In vivo models provide the ultimate test of a scaffold's ability to form functional bone in a physiological environment.
- Ectopic Bone Formation Model: A common model involves implanting the scaffold (e.g., 5 mm length constructs) subcutaneously in immuno-deficient mice (e.g., MF1 nu/nu) for 28 days. This model tests the intrinsic osteoinductivity of the material [90].
- Critical-Sized Defect Model: This orthotopic model, such as a rat condyle defect, assesses the ability of the scaffold to regenerate bone in a location that will not heal on its own. This is crucial for predicting clinical success [91].
Efficacy Endpoints and Analytical Methods:
- Micro-Computed Tomography (Micro-CT): This is the gold standard for 3D, quantitative analysis of bone formation in vivo. Scans are performed at a high resolution (e.g., 10-20 µm). Key parameters calculated include:
- Histomorphometry: Following scanning, explants are processed for undecalcified histology (e.g., embedded in resin and sectioned).
- Von Kossa or Goldner's Trichrome Stain: To identify mineralized bone matrix (stains black or green, respectively).
- Toluidine Blue: To visualize osteoid and cellular detail.
- Histomorphometric analysis quantifies the percentage of new bone area within the defect [90].
- Multimodal Imaging Workflow: A combined CT and MRI approach allows for simultaneous monitoring of new bone formation (via CT) and scaffold degradation (via MRI) over time [91].
Experimental Workflow for Bone Regeneration
The diagram below outlines a standard experimental workflow for evaluating a biomaterial in a bone regeneration model, from synthesis to final assessment.
The following tables consolidate key quantitative findings from recent studies in neural and bone regeneration, providing a reference for expected outcomes.
Table 1: In Vitro Efficacy Data from Neural and Bone Regeneration Studies
| Material System | Cell Type | Key Assay | Result | Citation |
|---|---|---|---|---|
| PCL/BG-B12 Nanofiber | PC12 | Neurite Length | Significant increase vs. control | [89] |
| PCL/BG-B12 Nanofiber | PC12 | NGF Production (ELISA) | Strikingly triggered NGF production | [89] |
| 3D Printed 5% GelMA | MSC | Viability & Proliferation | High cell viability and proliferation | [91] |
| ALG/ECM Hydrogel | HBMSC | Alizarin Red S | Mineralized nodule formation | [90] |
Table 2: In Vivo Efficacy Data from Neural and Bone Regeneration Studies
| Material System | Animal Model | Key Metric | Result | Citation |
|---|---|---|---|---|
| Pristine 3D GelMA | Rat Condyle Defect | Micro-CT / Histology | New bone formation, good integration, no fibrosis | [91] |
| ALG/ECM Hydrogel | Mouse Subcutaneous | Micro-CT / Histology | Dense, mineralized bone formation; host tissue invasion & vascularization | [90] |
| PHB Conduit | Human Nerve Injury | Clinical Trial (Double-blind) | Advantageous vs. epineural suturing | [88] |
| Stem Cell Therapy | Rat Nerve Injury | MRI Volumetry | Reduced neuronal cell death in DRG | [88] |
The Scientist's Toolkit: Essential Research Reagents and Materials
A successful research program in tissue engineering requires a suite of well-characterized reagents and materials. The following table details key components used in the studies cited in this guide.
Table 3: Essential Research Reagents and Materials for Tissue Engineering
| Item | Function/Description | Example Application |
|---|---|---|
| Gelatin Methacrylate (GelMA) | A photopolymerizable hydrogel derived from ECM; provides tunable mechanical properties and bioactivity. | 3D printable scaffold for bone regeneration [91]. |
| Poly-ε-caprolactone (PCL) | A biodegradable, synthetic polymer; offers excellent mechanical stability and processability. | Electrospun nanofiber scaffold for peripheral nerve guidance [89]. |
| Nerve Growth Factor (NGF) | A neurotrophic protein critical for neuronal survival, differentiation, and neurite outgrowth. | Stimulating neuronal differentiation of PC12 cells in vitro [89]. |
| Bone Morphogenetic Protein-2 (BMP-2) | A potent osteoinductive growth factor that stimulates bone formation. | Loading into slow-release microparticles to enhance osteogenesis in ALG/ECM hydrogels [90]. |
| Alginate/Bone ECM Hydrogel | A composite biomaterial combining alginate's structural properties with the bioactivity of decellularized bone ECM. | Highly osteoinductive scaffold for in vivo bone formation [90]. |
| Bioactive Glass (BG) | A surface-reactive ceramic material that bonds to bone and stimulates osteogenic activity. | Component of hybrid PCL nanofibers to enhance bioactivity [89]. |
| Stro-1 Antibody | A cell surface marker used for the identification and enrichment of skeletal stem cells from human bone marrow. | Isolation of human bone marrow stromal cells (HBMSCs) for bone regeneration studies [90]. |
| Poly(D,L-lactic-co-glycolic acid) (PLGA) | A biodegradable and biocompatible copolymer widely used for controlled drug delivery. | Fabrication of microparticles for spatiotemporal growth factor release in hydrogels [90]. |
Evaluating Drug Loading Capacity, Release Kinetics, and Targeting Efficiency
The development of advanced drug delivery systems (DDSs) represents a frontier in modern therapeutics, aiming to enhance drug efficacy while minimizing side effects. The performance of these systems hinges on three critical parameters: their drug loading capacity, the kinetics of drug release, and their ultimate targeting efficiency. Within the broader context of electrical, magnetic, and thermodynamic behaviors of materials, researchers can engineer "smart" carriers that respond to exogenous stimuli like magnetic fields or endogenous triggers such as pH changes. This guide provides a technical foundation for the rigorous evaluation of these key parameters, equipping researchers with the methodologies and analytical frameworks needed to advance targeted therapeutic interventions.
Quantitative Comparison of Drug Carrier Performance
The following tables summarize key performance metrics and material properties for a selection of advanced drug carriers, providing a benchmark for comparison and development.
Table 1: Performance Metrics of Selected Drug Carriers
| Drug Carrier Type | Model Drug | Max Drug Loading (%) | Release Profile (over 48h) | Targeting/Thermal Property |
|---|---|---|---|---|
| Fe₃O₄-PVA Magnetic Nanoparticle [92] | Adriamycin Hydrochloride (DOX·HCl) | 87% | 84% released | Thermal decomposition peak at 222°C; Magnetic targeting |
| Hollow Hydroxyapatite Microcapsules [93] | Multiple Drugs (Ivermectin, Ibuprofen, etc.) | 45% (v/v) | Accelerated dissolution | High specific surface area; Inhibited drug crystallization |
| Core-Multishell (CMS) Nanocarriers [94] | Dexamethasone | Quantified via a two-step dialysis method | Kinetics disentangled from membrane effects | Not Specified |
Table 2: Material Composition and Key Characteristics
| Drug Carrier Type | Core Material | Shell/Matrix Material | Key Functional Characteristics |
|---|---|---|---|
| Fe₃O₄-PVA Magnetic Nanoparticle [92] | Triiron Tetroxide (Fe₃O₄) | Polyvinyl Alcohol (PVA) | Hydrophilic shell; Magnetic core for targeting; Slow-release via polymer swelling |
| Hollow Hydroxyapatite Microcapsules [93] | Hydroxyapatite (Hollow Cavity) | Hydroxyapatite | Template Inverted Particle (TIP) structure; Self-loading mechanism into cavity |
| Stimuli-Responsive Microrobots [95] | Various (e.g., magnetic materials) | Smart Polymers (e.g., pH- or temperature-sensitive) | Remote actuation (e.g., magnetic fields); On-demand drug release via external stimuli |
Experimental Protocols for Core Evaluations
Protocol for Drug Loading Capacity Analysis
The drug loading capacity is a fundamental metric that defines the amount of a therapeutic agent a carrier can hold. A general protocol, adaptable for various nanocarriers, is outlined below.
- Step 1: Drug Loading Procedure. Incubate a known quantity of the dried drug carrier (e.g., Fe₃O₄-PVA nanoparticles) with a concentrated solution of the model drug (e.g., DOX·HCl) for a defined period, typically up to 48 hours, to reach equilibrium. [92] For hollow hydroxyapatite microcapsules (TIP), a "self-loading" mechanism facilitates efficient deposition of the drug into the internal cavity without complex procedures. [93]
- Step 2: Separation and Quantification. Separate the drug-loaded carriers from the free/unloaded drug solution via centrifugation or filtration. The concentration of the free drug in the supernatant is then quantified using analytical techniques such as UV-Vis spectroscopy or High-Performance Liquid Chromatography (HPLC). [92]
- Step 3: Calculation. The drug loading capacity can be calculated as a percentage using the formula:
(Total amount of drug added - Amount of free drug) / Mass of drug carrier × 100%. [92] For volumetric capacity, the result is expressed as(Volume of drug loaded / Total volume) × 100%. [93]
Protocol for Drug Release Kinetics
Evaluating release kinetics reveals how the drug is liberated from the carrier under specific conditions, which is critical for predicting in vivo performance.
- Step 1: Dialysis Setup. Place the drug-loaded carriers into a dialysis membrane (e.g., cellulose), which is then immersed in a release medium (e.g., phosphate-buffered saline at physiological pH or simulating target site pH). [94] The system is maintained at a constant temperature (e.g., 37°C) with continuous agitation.
- Step 2: Sampling and Measurement. At predetermined time intervals, withdraw aliquots of the release medium from outside the dialysis membrane and replace with fresh medium to maintain sink conditions. The concentration of the released drug in these samples is quantified using UV-Vis or HPLC. [92] [94]
- Step 3: Kinetic Modeling and Analysis. Plot the cumulative drug release versus time to generate the release profile. [92] A critical consideration is to account for the effect of the dialysis membrane itself on the observed release kinetics. A two-step approach is recommended:
- Calibration: Perform a control experiment to measure the diffusion rate of the free drug through the dialysis membrane in the absence of nanocarriers.
- Disentanglement: Use mathematical modeling to disentangle the kinetic properties of the dialysis membrane from the intrinsic release kinetics of the nanocarrier, providing a more accurate description of the carrier's performance. [94]
Protocol for Assessing Targeting Efficiency
Targeting efficiency evaluates a system's ability to accumulate and act at a specific site. For magnetically targeted systems, this can be assessed through in vitro and ex vivo models.
- Step 1: In Vitro Magnetic Targeting Models. Use microfluidic devices that simulate blood flow to test the retention of magnetic carriers (e.g., Fe₃O₄-based nanoparticles or microrobots) under an applied magnetic field. The percentage of carriers retained at the target "site" versus those washed away quantifies targeting efficacy under dynamic conditions. [95]
- Step 2: Ex Vivo and In Vivo Validation. In animal models, administer the drug carrier, often labeled with a fluorescent tag, with and without the application of an external magnetic field at the target tissue. Post-sacrifice, measure the drug concentration or fluorescence intensity in the target organ and non-target organs (e.g., liver, spleen). Targeting efficiency is calculated using indices such as the Drug Targeting Index (DTI):
(Drug concentration in target site with magnet) / (Drug concentration in target site without magnet)or by comparing the(AUC_target / AUC_non-target)ratios for targeted versus non-targeted systems. [92] [95] [96]
Visualization of Workflows and Material Behaviors
Experimental Workflow for Comprehensive Carrier Evaluation
The following diagram outlines the integrated experimental workflow for evaluating a smart drug delivery system from synthesis to performance analysis, highlighting the role of material properties.
Diagram Title: Workflow for Evaluating Smart Drug Carriers
Thermodynamic and Magnetic Behaviors in Smart Carriers
This diagram illustrates how external energy fields interact with the material properties of a carrier to control drug release, linking directly to electrical, magnetic, and thermodynamic principles.
Diagram Title: Material Responses to External Stimuli
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Reagents for Evaluating Drug Carriers
| Reagent / Material | Function in Research |
|---|---|
| Polyvinyl Alcohol (PVA) | A hydrophilic, biodegradable polymer used to form a shell on nanoparticles (e.g., around a Fe₃O₄ core) to improve biocompatibility and provide a matrix for controlled drug release. [92] |
| Triiron Tetroxide (Fe₃O₄) | A magnetic nanoparticle core that enables targeted delivery through application of an external magnetic field and can be used for hyperthermia therapy. [92] [95] |
| Hydroxyapatite Microcapsules | Inorganic, hollow carriers designed for high-capacity drug loading via a self-loading mechanism, particularly useful for creating orally disintegrating tablets. [93] |
| Dialysis Membranes (Cellulose) | A semi-permeable membrane used in a two-step experimental method to disentangle a drug carrier's intrinsic release kinetics from the diffusion properties of the membrane itself. [94] |
| Stimuli-Responsive Hydrogels | Smart polymer networks (e.g., sensitive to pH or temperature) that can be incorporated into microrobots or carriers to trigger drug release in response to specific biological cues or external stimuli. [95] |
First-Principles Calculations and DFT for Predicting Multiferroic and Thermoelectric Properties
First-principles calculations, particularly those based on density functional theory (DFT), have become indispensable tools in the computational prediction and characterization of material properties. These quantum mechanical methods enable researchers to solve the fundamental equations of materials systems without empirical parameters, providing powerful insights into electronic structure, magnetic ordering, and thermodynamic behavior. In the specialized domain of multiferroic and thermoelectric materials, DFT calculations offer unprecedented atomic-level understanding of structure-property relationships, guiding the rational design of next-generation functional materials for data storage, energy conversion, and spintronic applications [97].
The investigation of electrical, magnetic, and thermodynamic behaviors through computational approaches represents a paradigm shift in materials research. By employing advanced DFT methodologies, researchers can now predict diverse properties including electronic band structures, magnetic moments, elastic constants, piezoelectric responses, and thermoelectric coefficients before undertaking complex synthetic procedures. This technical guide provides a comprehensive framework for employing first-principles calculations to investigate multiferroic and thermoelectric materials, with detailed methodologies, data presentation standards, and visualization protocols tailored for research scientists and development professionals.
Theoretical Foundation
Fundamental Principles of Density Functional Theory
Density Functional Theory establishes that the ground-state energy of a quantum mechanical system is uniquely determined by its electron density, rather than the many-body wavefunction. This revolutionary approach reduces the computational complexity of solving the Schrödinger equation for many-electron systems from 3N dimensions (for N electrons) to just three spatial dimensions. The Hohenberg-Kohn theorems provide the mathematical foundation, while the Kohn-Sham equations offer a practical computational framework by replacing the original interacting electron system with an auxiliary non-interacting system that produces the same electron density [98].
The Kohn-Sham equations form the working heart of DFT calculations:
[\left[-\frac{\hbar^2}{2m}\nabla^2 + V{ext}(\mathbf{r}) + VH(\mathbf{r}) + V{XC}(\mathbf{r})\right]\psii(\mathbf{r}) = \epsiloni\psii(\mathbf{r})]
where (V{ext}) represents the external potential, (VH) the Hartree potential, and (V{XC}) the exchange-correlation potential that encapsulates all quantum mechanical many-body effects. The accuracy of DFT calculations critically depends on the approximation used for (V{XC}), with progressively sophisticated functionals including Local Density Approximation (LDA), Generalized Gradient Approximation (GGA), and meta-GGA approaches [99].
Advanced Computational Frameworks
For strongly correlated systems such as multiferroic oxides containing transition metal or rare-earth elements, standard DFT functionals often fail to adequately describe localized d and f electrons. The DFT+U method (Hubbard correction) introduces an on-site Coulomb interaction term to better capture electron localization and strong correlation effects, which is essential for accurately predicting electronic and magnetic properties [100]. The full-potential linearized augmented plane-wave (FP-LAPW) method, implemented in codes like WIEN2k, provides one of the most precise implementations for solving the Kohn-Sham equations, as it makes no shape approximations to the potential or electron density [99] [101].
The recent integration of machine learning approaches with DFT has created new opportunities for improving predictive accuracy, particularly for thermodynamic properties like formation enthalpies where traditional DFT has limitations. Neural network models trained on discrepancies between DFT-calculated and experimentally measured enthalpies can significantly enhance the reliability of phase stability predictions in complex multicomponent systems [102].
Computational Methodologies
Structural Modeling and Property Calculation
Table 1: Fundamental DFT Formalisms for Material Property Prediction
| Property Category | DFT Approach | Key Physical Quantities | Common Codes |
|---|---|---|---|
| Structural Properties | Geometry optimization via force minimization | Equilibrium lattice parameters, atomic positions, bond lengths | WIEN2k, VASP, Quantum ESPRESSO |
| Electronic Structure | GGA, GGA+U, mBJ for band gaps | Band structure, density of states (DOS), charge density | WIEN2k, Quantum ESPRESSO |
| Magnetic Properties | Spin-polarized DFT+U | Magnetic moments, exchange coupling, magnetic ordering | WIEN2k, VASP |
| Elastic Properties | Stress-strain relationship analysis | Elastic constants (Cij), bulk/shear modulus, Poisson's ratio | WIEN2k, VASP |
| Thermoelectric Properties | Boltzmann transport theory | Seebeck coefficient, electrical conductivity, power factor | BoltzTraP, WIEN2k |
| Thermodynamic Properties | Quasi-harmonic approximation (QHA) | Debye temperature, heat capacity, thermal expansion | Gibbs2, QHA codes |
The FP-LAPW method within the WIEN2k code has emerged as a particularly powerful approach for investigating complex materials. In this methodology, space is divided into non-overlapping atomic spheres and an interstitial region, with basis functions constructed differently in each region. Inside the atomic spheres, the wavefunctions are expanded in spherical harmonics, while in the interstitial region, plane waves are employed. This dual-basis approach allows for an accurate description of both rapidly oscillating wavefunctions near nuclei and smoother variations between atoms [99] [101].
For structural properties, calculations begin with geometry optimization to determine the equilibrium lattice parameters and atomic positions by minimizing the Hellmann-Feynman forces on atoms and the stress tensor components. The Perdew-Burke-Ernzerhof (PBE) parameterization of GGA typically provides better structural parameters than LDA, though often with underestimated band gaps. Electronic structure analysis involves computing band structures and density of states, with hybrid functionals or the Tran-Blaha modified Becke-Johnson (TB-mBJ) potential offering improved band gap accuracy for thermoelectric and optical property predictions [101].
Specialized Protocols for Multiferroic and Thermoelectric Materials
Table 2: Computational Parameters for Different Material Classes
| Material System | Recommended Functional | Key Properties | Special Considerations |
|---|---|---|---|
| Multiferroic Perovskites (e.g., YMnO₃, BiFeO₃) | GGA+U (U = 3-8 eV for d-electrons) | Polarization, magnetic ordering, band gap | Strong spin-orbit coupling may be needed for proper magnetoelectric response |
| Half-Metallic Ferromagnets (e.g., YMnS₃) | GGA+U (U = 3-5 eV) | Spin polarization, magnetic moment, Curie temperature | Metallic in one spin channel, insulating in another |
| Thermoelectric Oxides | GGA or mBJ for band gaps | Seebeck coefficient, electrical conductivity, electronic thermal conductivity | Boltzmann transport with constant relaxation time approximation |
| Skutterudites (e.g., RNi₄P₁₂) | GGA with spin polarization | Band degeneracy, carrier effective mass, phonon dispersion | Rattling phonon modes crucial for low thermal conductivity |
| Strained Systems | GGA with deformed lattice constants | Band structure deformation, effective mass changes | Strain engineering for ZT enhancement |
For multiferroic materials, computational protocols must address both ferroelectric and magnetic properties simultaneously. The Berry phase approach or modern theory of polarization is employed to calculate spontaneous polarization, while magnetic properties are determined through spin-polarized calculations with appropriate U parameters. For YMnO₃, a prototypical multiferroic, calculations reveal a polar P6₃cm symmetry with cation displacements along the c-axis generating spontaneous polarization, and G-type antiferromagnetic ordering as the ground state [99] [103].
Thermoelectric property prediction involves a multi-step process: First, a precise electronic structure calculation provides the band energies. These are then used as input to the Boltzmann transport equation within the constant relaxation time approximation, typically implemented in codes like BoltzTraP. This approach computes the Seebeck coefficient, electrical conductivity, and electronic thermal conductivity as functions of chemical potential and temperature [98] [101]. The thermoelectric figure of merit ZT is subsequently evaluated from these transport coefficients.
Computational Workflow for Multiferroic and Thermoelectric Materials Prediction
Case Studies and Applications
Multiferroic Perovskite Oxides
YMnO₃ represents a classic hexagonal multiferroic system where first-principles calculations have revealed fundamental structure-property relationships. DFT investigations demonstrate that the polar P6₃cm phase is more stable than the centrosymmetric structure, with calculated lattice parameters (a = 3.264 Å, c = 11.820 Å) showing excellent agreement with experimental values. The electronic structure reveals a semiconductor character with a direct band gap of approximately 1.88 eV, while magnetic property calculations confirm antiferromagnetic ordering with a significant magnetic moment primarily localized on Mn atoms [99] [103].
Doping strategies can effectively tune the properties of multiferroic materials. Fe-doped YMnO₃ exhibits remarkable modifications in electronic and magnetic behavior. DFT calculations show that 25% Fe doping reduces the band gap to 1.19 eV, enhancing visible light absorption for potential photovoltaic applications. Additionally, Fe doping reduces spin frustration, induces net magnetization, and improves dielectric response with increased static dielectric constant. These calculated predictions provide a foundation for tailoring multiferroic oxides for specific device applications [103].
Thermoelectric Materials
The lanthanum monopnictides LaX (X = P, As) demonstrate how strain engineering can dramatically enhance thermoelectric performance. DFT calculations combined with Boltzmann transport theory reveal that under optimal conditions of 2% isotropic tensile strain and carrier concentration n = 3×10²⁰ cm⁻³, LaP achieves a figure of merit ZT > 2 at 1200 K. This represents a 90% enhancement compared to the unstrained value, highlighting the power of computational guidance for performance optimization [98].
Filled skutterudites RNi₄P₁₂ (R = Sm, Eu) represent another class where DFT calculations provide crucial insights. Electronic structure calculations reveal dense energy bands near the Fermi energy originating from rare-earth and Ni atoms, suggesting promising thermoelectric performance. The computed transport properties show high electrical conductivity coupled with moderate Seebeck coefficients, while the complex crystal structure with filler atoms enables phonon scattering that reduces lattice thermal conductivity - a key requirement for high ZT values [101].
Half-Metallic Ferromagnetic Materials
YMnS₃ exemplifies how DFT+U calculations can predict novel functional properties. Standard GGA calculations suggest metallic behavior, but the incorporation of Hubbard U (U = 3 eV) reveals a transition to half-metallic ferromagnetic character. In this state, the material exhibits metallic behavior in one spin channel while being semiconducting in the other, resulting in 100% spin polarization at the Fermi level. The calculated total magnetic moment is approximately 4.02 μB, and the half-metallic band gap increases with higher U values, making this material promising for spintronic applications [100].
Research Reagent Solutions
Table 3: Essential Computational Tools and Parameters
| Resource Category | Specific Tools/Parameters | Function/Role | Application Context |
|---|---|---|---|
| DFT Software Packages | WIEN2k, VASP, Quantum ESPRESSO | Solving Kohn-Sham equations with various approximations | Core computational engine for property prediction |
| Exchange-Correlation Functionals | PBE-GGA, PBEsol, SCAN, HSE06 | Approximating quantum mechanical exchange-correlation effects | Determining accuracy for different material classes |
| Hubbard U Parameters | U = 3-5 eV (Mn 3d), U = 6-8 eV (Fe 3d) | Correcting self-interaction error in strongly correlated systems | Transition metal oxides, rare-earth compounds |
| Transport Property Codes | BoltzTraP, BoltzWann, AMSET | Solving Boltzmann transport equation | Thermoelectric coefficient calculation |
| Structural Analysis Tools | VESTA, GDIS, J-ICE | Visualization and analysis of crystal structures | Pre- and post-processing of DFT calculations |
| Pseudopotentials/PAW Sets | Projector Augmented-Wave (PAW) potentials, pseudopotentials | Representing core-valence electron interactions | Basis set construction for plane-wave codes |
Data Presentation and Analysis
The comprehensive nature of first-principles investigations necessitates systematic organization of computational findings. Effective data presentation enables clear comparison between different materials systems and computational approaches, facilitating knowledge transfer and experimental validation.
Table 4: Comparative DFT Results for Selected Multiferroic and Thermoelectric Materials
| Material | DFT Method | Key Predicted Properties | Application Potential |
|---|---|---|---|
| YMnO₃ | GGA+U (U = 5-7 eV) | Band gap: 1.88 eV, AFM ordering, spontaneous polarization | Multiferroic memory devices, sensors |
| Fe-doped YMnO₃ | GGA+U (U = 5 eV) | Reduced band gap: 1.19 eV, enhanced dielectric response | Photovoltaics, optoelectronics |
| YMnS₃ | GGA+U (U = 3 eV) | Half-metallic gap: ~0.5 eV, magnetic moment: 4.02 μB | Spintronics, data storage |
| GaMnO₃ | GGA/PBE | c/a = 3.62, piezoelectric coefficient ~4.65 pm/V | Piezoelectric sensors, energy harvesting |
| LaP | GGA with scissor correction | ZT > 2 at 1200 K with 2% tensile strain | High-temperature thermoelectric generator |
| SmNi₄P₁₂ | GGA, GGA+mBJ | Metallic behavior, high density of states at EF | Thermoelectric power generation |
First-principles calculations based on density functional theory have matured into powerful predictive tools for designing and optimizing multiferroic and thermoelectric materials. The methodologies outlined in this technical guide provide a comprehensive framework for investigating structural, electronic, magnetic, elastic, and transport properties from quantum mechanical principles. As computational approaches continue to evolve through advanced exchange-correlation functionals, machine learning corrections, and high-throughput screening protocols, their integration with experimental materials research will undoubtedly accelerate the discovery of novel multifunctional materials with tailored properties for next-generation technological applications. The case studies presented demonstrate the remarkable success of these computational strategies in predicting and explaining complex materials behavior across diverse material classes, establishing first-principles calculations as an essential component of modern materials physics and chemistry research.
Benchmarking Against Clinically Approved Formulations and Established Therapeutic Modalities
Benchmarking new therapeutic candidates against established standards is a critical process in pharmaceutical development, ensuring that new treatments offer meaningful improvements over existing options. This guide frames this essential activity within the broader context of materials science, particularly through the lens of electrical, magnetic, and thermodynamic behaviors of biological and synthetic materials. The properties of drug formulations—including their electrical surface characteristics, magnetic susceptibility in targeted delivery systems, and thermodynamic stability—directly influence their biological performance, safety, and efficacy. Modern drug development has seen a pronounced shift toward novel therapeutic modalities, which now represent approximately 60% of the total pharmaceutical pipeline value, projected at $197 billion in 2025 [104]. This document provides researchers and drug development professionals with a technical framework for conducting rigorous, materials-informed benchmarking studies, complete with standardized protocols and analytical methodologies for cross-modal comparison.
The Evolving Therapeutic Landscape: Key Modalities for Benchmarking
The drug development landscape is no longer dominated by small molecules alone. A diverse array of modality classes, each with distinct materials properties and performance characteristics, now comprises the competitive landscape. Understanding these classes is fundamental to designing appropriate benchmarking studies.
Established Modalities Showing Robust Growth
- Monoclonal Antibodies (mAbs): As one of the oldest new modalities, the mAbs clinical pipeline remains the largest and continues to expand with 7% more clinical-stage products than the previous year. Their growth is now extending into new therapeutic areas like neurology, rare diseases, and cardiovascular diseases beyond their traditional strongholds in oncology and immunology [104].
- Antibody-Drug Conjugates (ADCs): ADCs have demonstrated a 40% growth in expected pipeline value over the past year, fueled by approvals of targeted therapies like Datroway (datopotamab deruxtecan) for breast cancer [104] [105].
- Bispecific Antibodies (BsAbs): Forecasted pipeline revenue for BsAbs has risen 50% in the past year. A significant driver is the CD3 T-cell engager mechanism of action, which is utilized in seven of the top ten BsAbs ranked by forecasted 2030 revenue [104].
- Recombinant Proteins and Peptides: This category has experienced an 18% revenue increase, largely propelled by the massive success of GLP-1 agonists such as Mounjaro, Zepbound, and Wegovy [104].
Emerging Modalities with Mixed Performance
- Cell Therapies: CAR-T therapies continue to show strong growth and patient impact in hematology, though results in solid tumors and autoimmune diseases have been mixed. Other emerging cell therapies like TCR-T, TIL, and CAR-NK have faced challenges related to clinical delays, high manufacturing costs, and limited adoption, leading to a more conservative revenue outlook [104].
- Gene Therapies: Growth in this sector has stagnated due to safety issues, halted trials, and regulatory scrutiny. Commercialization challenges have also emerged, with some companies halting launches due to limited interest from patients and physicians despite technical approval [104].
- Nucleic Acid Therapies: This category shows divergent trajectories. DNA/RNA therapies and RNAi are on a steady upward path with a 65% and 27% increase in projected revenue, respectively. In contrast, mRNA pipeline value continues to decline significantly as the market adjusts post-COVID-19 pandemic [104].
Table 1: Select FDA-Approved Novel Therapies in 2025 Serving as Benchmarking Candidates
| Drug (Brand Name) | Therapeutic Area | Modality | Key Indication | Approval Date |
|---|---|---|---|---|
| Hernexeos (zongertinib) [106] | Oncology | Small Molecule | NSCLC with HER2 mutations | 2025-08-08 |
| Dawnzera (donidalorsen) [106] | Rare Disease | Nucleic Acid | Hereditary angioedema prevention | 2025-08-21 |
| Lynozyfic (linvoseltamab-gcpt) [106] | Oncology | Bispecific Antibody | Relapsed/Refractory Multiple Myeloma | 2025-07-02 |
| Datroway (datopotamab deruxtecan) [105] | Oncology | Antibody-Drug Conjugate | HR-positive, HER2-negative breast cancer | 2025-01-17 |
| Brinsupri (brensocatib) [106] | Pulmonology | Small Molecule | Non-cystic fibrosis bronchiectasis | 2025-08-12 |
| Qfitlia (fitusiran) [105] | Hematology | RNAi Therapeutic | Hemophilia A or B | 2025-03-28 |
Materials Science Fundamentals for Pharmaceutical Benchmarking
The performance of any drug formulation is intrinsically linked to its materials properties. A systematic benchmarking process must therefore incorporate an analysis of these fundamental characteristics.
The Ashby Methodology for Material Selection in Drug Formulations
The Ashby materials selection chart method provides a powerful graphical tool for the initial screening of materials based on multiple properties simultaneously. This approach is ideal for selecting excipients, polymer matrices for controlled release, or material platforms for medical devices. The charts plot one material property against another (e.g., Young's Modulus vs. Density) on logarithmic scales, allowing for the quick visualization and comparison of different material families [107].
Key performance indices relevant to drug formulation include:
- E/ρ (Specific Stiffness): Critical for lightweight, stiff implantable drug reservoirs.
- E^1/3^/ρ: Important for panels or structural components in bend-loaded configurations.
- σ/ρ (Specific Strength): Essential for materials under tensile load in wearable delivery systems [107].
This method is systematic and impartial, focusing on the product design objectives. It allows researchers to eliminate unsuitable materials quickly and identify a subset of candidates that maximize component performance, which can later be refined using Multi-Criteria Decision-Making (MCDM) techniques for more complex constraints [107].
Critical Material Properties in Therapeutic Performance
- Electrical Properties: Surface charge (zeta potential) of nanoparticles and lipid complexes dictates their colloidal stability, interaction with biological membranes, and cellular uptake efficiency. The recent discovery that magnetic waves (magnons) in antiferromagnetic materials can generate electric signals points to future applications where magnetic and electric systems are merged for controlled drug release or sensing [12].
- Thermodynamic Properties: The glass transition temperature (T~g~) of polymer excipients determines the physical stability of amorphous solid dispersions. Crystallization energy and enthalpy of dissolution are critical predictors of solubility and release kinetics. These properties must be benchmarked against those of established formulations to ensure equivalent or superior stability.
- Magnetic Properties: Magnetic susceptibility is leveraged in targeted delivery systems and diagnostic imaging agents. Research into magnons has revealed they can move at terahertz frequencies—around a thousand times faster than magnetic waves in conventional materials—suggesting a path toward ultrafast, low-power control systems for implanted therapeutic devices [12].
Experimental Framework for Benchmarking Studies
A robust benchmarking study requires standardized protocols to ensure reproducibility and meaningful comparison. The following section outlines detailed methodologies.
Protocol for Materials Property Characterization
Objective: To quantitatively compare the key physical properties of a novel formulation against a clinically approved benchmark.
Background: This protocol provides a workflow for the systematic characterization of a drug formulation's critical electrical, magnetic, and thermodynamic properties. The resulting data serves as the foundation for a materials-informed benchmarking assessment.
Diagram Title: Materials Property Characterization Workflow
Materials and Reagents:
- Test formulation and clinically approved benchmark
- Appropriate dissolution media (e.g., phosphate-buffered saline, simulated gastric/intestinal fluid)
- Reference standard for analytical calibration
Procedure:
- Sample Preparation:
- Prepare identical sample sizes (e.g., 10-50 mg) of both test and benchmark formulations.
- For solutions/suspensions, prepare at a standard concentration (e.g., 1 mg/mL) in relevant buffer.
- Allow all samples to equilibrate to standard laboratory temperature (e.g., 25°C) for 30 minutes before analysis.
Thermodynamic Analysis (DSC/TGA):
- Load 5-10 mg of sample into a hermetically sealed pan for Differential Scanning Calorimetry (DSC).
- Run a heat-cool-heat cycle from -50°C to 250°C at a standard heating rate (e.g., 10°C/min).
- Record the glass transition temperature (T~g~), melting point (T~m~), and any crystallization events.
- Concurrently, load 10-20 mg for Thermogravimetric Analysis (TGA) and heat from room temperature to 600°C to determine thermal stability and residual solvent content.
Electrical Property Analysis:
- Disperse the formulation in a suitable aqueous buffer at a standard concentration.
- Using a Zeta Potential analyzer, perform a minimum of 3 runs to determine the average surface charge (Zeta Potential) and particle size distribution.
- Measure the conductivity of the bulk solution using a calibrated conductivity meter.
Magnetic Property Analysis (If Applicable):
- For formulations with magnetic components, use a Vibrating Sample Magnetometer (VSM).
- Apply a variable magnetic field (e.g., -20 kOe to +20 kOe) at room temperature to generate a magnetization curve.
- Determine the saturation magnetization (M~s~) and coercivity (H~c~).
Troubleshooting Tips:
- If DSC shows no thermal events, confirm the sample is not fully crystalline or verify pan sealing.
- For low Zeta Potential values, ensure the dispersion medium has the appropriate ionic strength and check for electrode contamination.
- High TGA weight loss below 100°C may indicate absorbed moisture; consider pre-drying the sample.
The SIRO Model for Protocol Representation
To standardize the reporting and retrieval of benchmarking protocols, the Sample, Instrument, Reagent, Objective (SIRO) model is recommended. This minimal information model, analogous to the PICO model in evidence-based medicine, facilitates the semantic representation of experimental protocols, enabling better classification, sharing, and reproducibility [108].
- Sample: The material being tested (e.g., novel lipid nanoparticle formulation, benchmark ADC).
- Instruments: The key equipment used for characterization (e.g., DSC, Zeta Potential Analyzer, VSM).
- Reagents: All critical chemicals and buffers used in the analysis.
- Objective: The specific goal of the benchmarking protocol (e.g., "Compare thermal stability of Formulation A vs. Benchmark B").
Adopting the SIRO model ensures that the core elements of a protocol are machine-readable and searchable, addressing a major challenge in the reproducibility of biomedical research [108].
Table 2: Essential Research Reagent Solutions for Benchmarking Studies
| Reagent/Material | Function in Benchmarking | Example Application |
|---|---|---|
| Phosphate Buffered Saline (PBS) | Provides a physiologically relevant dispersion medium for stability and zeta potential tests. | Simulating in vivo conditions for nanoparticle characterization. |
| Reference Standard | Serves as a calibrated benchmark for analytical method validation and direct comparison. | Quantifying drug loading and purity against a known standard. |
| Hermetic Sealed DSC Pans | Ensures an isolated environment for controlled thermal analysis, preventing moisture loss. | Accurate measurement of glass transition and melting temperatures. |
| Standardized Buffer Solutions | Used for calibrating pH and conductivity meters to ensure measurement accuracy. | Preparing solutions for zeta potential and dissolution testing. |
| Model Lipid Membranes | Simulate biological barriers for uptake and permeability studies. | Benchmarking the membrane interaction potential of a new formulation. |
Data Analysis and Interpretation
Constructing Ashby Charts for Formulation Selection
After data collection, plot the properties of the test and benchmark formulations on Ashby-style charts. For instance:
- Create a chart of Enthalpy of Dissolution vs. Zeta Potential to identify formulations with optimal solubility and stability.
- Plot Glass Transition Temperature (T~g~) vs. Saturation Magnetization for magnetic-responsive formulations.
Interpreting these charts involves:
- Defining Constraints: Draw vertical or horizontal lines representing minimum or maximum acceptable values for critical properties.
- Plotting Performance Indices: Draw lines of constant performance indices (e.g., E/ρ) to identify materials that maximize a desired function.
- Identifying the Search Region: The subset of materials that satisfy all constraints and appear above the performance index line represents the viable candidates for further development [107].
Statistical and Comparative Reporting
- Perform statistical analysis (e.g., t-tests, ANOVA) to determine if differences in material properties between the test and benchmark are significant.
- Report results in a standardized table comparing the test formulation against the benchmark across all measured properties, highlighting statistically significant improvements (p < 0.05).
- Discuss the potential clinical implications of the observed differences in material properties. For example, a higher T~g~ may predict better long-term physical stability, while a more negative zeta potential may suggest increased cellular uptake.
Effective benchmarking of new drug formulations requires moving beyond simple biological activity comparisons to embrace a holistic, materials-centric approach. By systematically characterizing and comparing the electrical, magnetic, and thermodynamic properties of new candidates against clinically approved benchmarks, researchers can gain deeper insights into performance and potential failure modes early in the development process. The integration of the Ashby selection methodology for rational material choice and the SIRO model for standardized protocol reporting creates a robust, reproducible, and data-driven framework for therapeutic advancement. As the industry continues to evolve toward more complex modalities like ADCs, bispecifics, and nucleic acid therapies, grounded in the fundamental principles of materials science, this rigorous benchmarking paradigm will be indispensable for developing the next generation of transformative medicines.
Conclusion
The synergistic interplay of electrical, magnetic, and thermodynamic properties in advanced materials opens unprecedented avenues for biomedical innovation. Foundational research into multiferroics and magnetic nanomaterials has established a robust platform for developing targeted drug delivery systems, responsive tissue scaffolds, and novel cancer therapies. Methodological advances in synthesis and functionalization now enable precise control over material behavior in biological environments, while computational tools and high-throughput screening accelerate the discovery of next-generation materials. Overcoming persistent challenges in biocompatibility, manufacturing scalability, and rigorous preclinical validation remains crucial for clinical translation. Future directions should focus on multifunctional theragnostic platforms, AI-driven material design, and leveraging emerging phenomena like altermagnetism and topological magnetism. For researchers and drug development professionals, mastering these coupled behaviors is key to creating more effective, personalized, and minimally invasive medical treatments.
References
- 1. Magnetoelectric phenomena and devices - PMC [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3895980/]
- 2. Advances in magnetoelectric | Nature Materials multiferroics [https://www.nature.com/articles/s41563-018-0275-2?error=cookies_not_supported&code=e4ada77a-4c19-4f6d-93ca-007e6d1023a0]
- 3. and Multiferroics : thin films and nanostructures magnetoelectrics [https://research.utwente.nl/en/publications/multiferroics-and-magnetoelectrics-thin-films-and-nanostructures]
- 4. of multicaloric effects in Thermodynamics ... multiferroic materials [https://pubmed.ncbi.nlm.nih.gov/27402925/]
- 5. Recent advances in ferroic, multiferroic, and magnetoelectrics materials and phenomena - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/B9780443186066000125]
- 6. of Bio- Classification | Encyclopedia MDPI Magnetic Nanoparticles [https://encyclopedia.pub/entry/46632]
- 7. Promising biomedical applications using superparamagnetic... [https://eurjmedres.biomedcentral.com/articles/10.1186/s40001-025-02696-z]
- 8. Joule Heating's Role in Electrical Behavior of Materials - Simple Science [https://scisimple.com/en/articles/2025-07-16-joule-heatings-role-in-electrical-behavior-of-materials--aky5yyl]
- 9. Thermodynamic description for magneto-plastic coupling in electrical steel sheets - ScienceDirect [https://www.sciencedirect.com/science/article/pii/S0304885324001379]
- 10. Energy-based ferromagnetic material model with magnetic anisotropy - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S0304885316309404]
- 11. A Comprehensive Review of Recent Advances in Perovskite Materials: Electrical, Dielectric, and Magnetic Properties [https://www.mdpi.com/2304-6740/13/3/67]
- 12. Breakthrough links magnetism and electricity for faster tech | ScienceDaily [https://www.sciencedaily.com/releases/2025/11/251104094141.htm]
- 13. Magnetic materials - Latest research and news | Nature [https://www.nature.com/subjects/magnetic-materials]
- 14. in a frustrated Thermodynamic ... phase transitions magnetic [https://www.nature.com/articles/ncomms9278?error=cookies_not_supported&code=e0f52ac7-5ea7-477a-9741-832cb049444c]
- 15. Advances in CrSBr Research Properties - Simple Science Magnetic [https://scisimple.com/en/articles/2025-07-06-research-advances-in-crsbr-magnetic-properties--a30j6d4]
- 16. Pathways to Bubble and Skyrmion Lattice Formation in Fe/Gd Multilayers [https://arxiv.org/html/2501.18459v1]
- 17. How to write a materials and methods section of a scientific article? - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC4548564/]
- 18. Experimental reporting [https://www.rsc.org/publishing/publish-with-us/publish-a-journal-article/experimental-reporting]
- 19. Reporting standards and availability of data, materials, code and protocols | Nature Portfolio [https://www.nature.com/nature-portfolio/editorial-policies/reporting-standards]
- 20. Ion and Proton Transport In Aqueous/Nonaqueous Acidic Ionic Liquids for Fuel-Cell Applications—Insight from High-Pressure Dielectric Studies - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC8289238/]
- 21. Hydrogen Bond Strength Dictates the Rate-Limiting Steps of Diffusion ... [https://arxiv.org/html/2503.07291v1]
- 22. Mechanism of Efficient Proton Conduction in Diphosphoric Acid Elucidated via First-Principles Simulation and NMR - PubMed [https://pubmed.ncbi.nlm.nih.gov/26633234/]
- 23. The persistence of memory in ionic probed by... | Nature conduction [https://www.nature.com/articles/s41586-023-06827-6?error=cookies_not_supported&code=6cbc2b21-6812-46ed-873a-ec3c438ee5d3]
- 24. Structural origin of proton mobility in a protic ionic liquid/imidazole mixture: insights from computational and experimental results - PubMed [https://pubmed.ncbi.nlm.nih.gov/27499376/]
- 25. Frontiers | Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications [https://www.frontiersin.org/journals/chemistry/articles/10.3389/fchem.2021.629054/full]
- 26. Magnetic Nanoparticles: Advances in Synthesis, Sensing, and Theragnostic Applications [https://www.mdpi.com/2312-7481/11/2/9]
- 27. Magnetic Nanoparticles: A Review on Synthesis, Characterization, Functionalization, and Biomedical Applications - PubMed [https://pubmed.ncbi.nlm.nih.gov/37732364/]
- 28. Preparation and characterization of superparamagnetic magnetite... [https://mjfas.utm.my/index.php/mjfas/article/view/1224]
- 29. Room Temperature Co-Precipitation Synthesis of Magnetite Nanoparticles in a Large pH Window with Different Bases - PMC [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5452734/]
- 30. Magnetic Nanoparticles: The Simulation of Thermodynamic Properties | SpringerLink [https://link.springer.com/chapter/10.1007/3-540-44946-9_49]
- 31. Frontiers | Effect of precipitating agent, N2 gas, extract volume and pH on the magnetic properties of magnetite nanoparticles by green synthesis from aqueous pomegranate peel extract [https://www.frontiersin.org/journals/chemistry/articles/10.3389/fchem.2024.1413077/full]
- 32. Nanoparticle Surface : How to Improve... Functionalization [https://pmc.ncbi.nlm.nih.gov/articles/PMC7726445/]
- 33. Biomedical Coatings : Surface Engineering For Enhanced... [https://journals.stmjournals.com/jotcsta/article=2025/view=201762/]
- 34. sciencedirect.com/topics/materials-science/ surface - functionalization [https://www.sciencedirect.com/topics/materials-science/surface-functionalization]
- 35. Exploring Structural, Optoelectronic, Phonon, Spintronic, and Thermodynamic Properties of Novel Full-Heusler Compounds TiMCu2 (M = Al, Ga, In): Eco-Friendly Materials for Next-Generation Renewable Energy Technologies [https://www.mdpi.com/2073-4352/15/10/876]
- 36. Advanced thermal and magnetic materials for high-power and high-temperature applications: a comprehensive review | Discover Materials [https://link.springer.com/article/10.1007/s43939-025-00305-8]
- 37. An updated review and recent advancements in carbon-based... [https://pubmed.ncbi.nlm.nih.gov/39033875/]
- 38. Biocompatible functionalization of polymersome surfaces : a new... [https://pureportal.strath.ac.uk/en/publications/biocompatible-functionalization-of-polymersome-surfaces-a-new-app]
- 39. Recent Advances on Magnetic Sensitive Hydrogels in Tissue Engineering - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC7068712/]
- 40. Magnetic-responsive hydrogels: From strategic design to biomedical applications - PubMed [https://pubmed.ncbi.nlm.nih.gov/34097923/]
- 41. Geometrothermodynamic description of magnetic materials [https://arxiv.org/html/2402.17782]
- 42. Vers une nouvelle approche CALPHAD du magnétisme dans les métaux et alliages - PolyPublie [https://publications.polymtl.ca/66246/]
- 43. Polysaccharide-based magnetically polyelectrolyte... responsive [https://www.jmst.org/EN/10.1016/j.jmst.2017.10.003]
- 44. International Baltic Conference on Magnetism – 2025 ... Research [https://smba.science/ibcm-2025/]
- 45. Recent Advances in the Development of Drug Delivery Applications of Magnetic Nanomaterials - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC10383769/]
- 46. Magnetic Nanoparticles and Drug Delivery Systems for Anti-Cancer Applications: A Review [https://www.mdpi.com/2079-4991/15/4/285]
- 47. Micro/Nanosystems for Magnetic Targeted Delivery of Bioagents - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC9230665/]
- 48. Use of magnetic fields and nanoparticles to trigger and... drug release [https://pubmed.ncbi.nlm.nih.gov/31241251/]
- 49. Multifunctional Magnetic Nanoparticles for Targeted Delivery... Drug [https://link.springer.com/article/10.1007/s10439-025-03712-3]
- 50. Nanoparticles for Magnetic Delivery in a Mouse Model... Targeted Drug [https://jns.kashanu.ac.ir/article_114509.html]
- 51. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC7069093/]
- 52. Frontiers | Advances in magnetic induction hyperthermia [https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2024.1432189/full]
- 53. Treatment as a Promising Hyperthermia - Anti Strategy... Cancer [https://pmc.ncbi.nlm.nih.gov/articles/PMC9030926/]
- 54. for Targeting Hyperthermia and Cancer Stem Cancer : Insights... Cells [https://link.springer.com/article/10.1007/s12015-024-10736-0]
- 55. Enhanced Multimodal Effect of Chemotherapy, Hyperthermia and Magneto-Mechanic Actuation of Silver-Coated Magnetite on Cancer Cells [https://www.mdpi.com/2079-6412/13/2/406]
- 56. -field-controlled Magnetic of mechanical -sensitive... behavior magneto [https://link.springer.com/article/10.1007/s00419-018-1477-4]
- 57. Magnetite Nanoparticles in Magnetic Hyperthermia and Cancer Therapies: Challenges and Perspectives - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC9182445/]
- 58. Mapping intracellular thermal response of cancer cells to magnetic hyperthermia treatment - PubMed [https://pubmed.ncbi.nlm.nih.gov/32766635/]
- 59. : Magnetic generation and... nanoparticles reactive oxygen species [https://link.springer.com/article/10.1007/s11051-017-3943-2]
- 60. Magnetic Nanoparticles: Surface Effects and Properties Related to Biomedicine Applications - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC3856004/]
- 61. Thermodynamics at the nanoscale: A new approach to the investigation of unique physicochemical properties of nanomaterials - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S0927796X14000242]
- 62. Enhanced cytotoxicity caused by AC magnetic field for polymer... [https://pubmed.ncbi.nlm.nih.gov/33421925/]
- 63. Uncovering a possible role of reactive in... oxygen species [https://www.nature.com/articles/s41598-020-70067-1?error=cookies_not_supported&code=ed338379-c73b-4a28-a3cb-bd610e2bf3b5]
- 64. Properties of Rutile TiO2 Nanoparticles | IJN Optimizing Magnetic [https://www.dovepress.com/synthesizing-and-optimizing-rutile-tio2-nanoparticles-for-magnetically-peer-reviewed-fulltext-article-IJN]
- 65. Exploring the Enhancement of Exchange Bias in Innovative... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10005359/]
- 66. Microstructural, Morphological, and Magnetic Effects of NiFe2O4 Shell ... [https://colab.ws/articles/10.3390/magnetochemistry11010002]
- 67. Discovery of new materials using combinatorial synthesis and high-throughput characterization of thin-film materials libraries combined with computational methods | npj Computational Materials [https://www.nature.com/articles/s41524-019-0205-0]
- 68. Using scalable computer vision to automate high - throughput ... [https://www.nature.com/articles/s41467-024-48768-2?error=cookies_not_supported]
- 69. CheatSheet Material properties [https://www.luisllamas.es/en/material-properties-cheatsheet/]
- 70. Thermoelectric Materials : Properties & Applications [https://mindmapai.app/mind-mapping/thermoelectric-materials-1]
- 71. Visualizing Quantitative Data: Best Experience [https://powerdrill.ai/blog/visualizing-quantitative-data-best-experience]
- 72. Top 6 Visualizations for Quantitative Data Analysis Methods - [https://chartexpo.com/blog/quantitative-data-analysis-methods]
- 73. Frontiers | Recent advances in surface decoration of nanoparticles in drug delivery [https://www.frontiersin.org/journals/nanotechnology/articles/10.3389/fnano.2024.1456939/full]
- 74. Dual character of surface on SN38 prodrug... engineering [https://pubmed.ncbi.nlm.nih.gov/40976820/]
- 75. of Exosomes and Biodistribution Strategies for Targeted... Engineering [https://link.springer.com/article/10.1007/s13770-021-00361-0]
- 76. Revolutionizing Drug Delivery: The Impact of Advanced Materials Science and Technology on Precision Medicine - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC11944361/]
- 77. Frontiers | Recent advances in mesoporous silica nanoparticle: synthesis, drug loading, release mechanisms, and diverse applications [https://www.frontiersin.org/journals/nanotechnology/articles/10.3389/fnano.2025.1564188/full]
- 78. for Computations – discovery, materials and the role of data design [https://www.european-mrs.com/computations-materials-%E2%80%93-discovery-design-and-role-data-emrs]
- 79. of Catalysts with Computational ... Design Experimental Validation [https://pubmed.ncbi.nlm.nih.gov/39502804/]
- 80. Materials Science in the AI age: high-throughput library generation, machine learning and a pathway from correlations to the underpinning physics - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC7067066/]
- 81. inverse Experimentally of FeNiCrCoCu MPEAs and... validated design [https://www.nature.com/articles/s41524-025-01600-x?error=cookies_not_supported&code=bec9191e-cb33-4dfe-bfc6-effb8c969ab2]
- 82. sciencedirect.com/topics/ materials -science/ specific - absorption - rate [https://www.sciencedirect.com/topics/materials-science/specific-absorption-rate]
- 83. Quantitative Analysis of the Specific Dependence... Absorption Rate [https://pubmed.ncbi.nlm.nih.gov/33096631/]
- 84. of Saturation for Soft and Hard... | CoLab Specific Absorption Rate [https://colab.ws/articles/10.3390/magnetochemistry6020023]
- 85. Pitfalls and Challenges in Specific ... | Preprints.org Absorption Rate [https://www.preprints.org/manuscript/202501.2101/v1]
- 86. Conference-service [https://conference-service.com/conferences/magnetism.html]
- 87. Exploration of biomaterial and stem cell-based strategies for promoting... [https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-025-07266-9]
- 88. Laboratory of Neural Repair and Cellular Therapy [https://www.umu.se/en/research/groups/laboratory-of-neural-repair-and-cellular-therapy/]
- 89. evaluation of the nanofibers developed for peripheral nerve... In vitro [https://link.springer.com/article/10.1007/s10561-025-10201-3]
- 90. of In in... | PLOS One Vivo Assessment Bone Regeneration [https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0145080]
- 91. and In of a 3D printable gelatin methacrylate... vitro in vivo assessment [https://pubmed.ncbi.nlm.nih.gov/35388573/]
- 92. Preparation and Thermal Decomposition Kinetics of a New Type of... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7860512/]
- 93. mechanism of hollow hydroxyapatite microcapsules Drug loading [https://pubmed.ncbi.nlm.nih.gov/40518056/]
- 94. General method for the quantification of drug loading and release kinetics of nanocarriers - PubMed [https://pubmed.ncbi.nlm.nih.gov/28017797/]
- 95. Smart Drug Delivery for Targeted Therapeutics via... | IntechOpen [https://www.intechopen.com/chapters/1184157]
- 96. , Electrical , photomechanical and cavitational waves to... magnetic [https://pubmed.ncbi.nlm.nih.gov/24801250/]
- 97. [0904.3736] First Principles Studies of Multiferroic Materials [https://arxiv.org/abs/0904.3736]
- 98. First-Principles Modeling of Mechanical Behavior and Thermoelectric Property Under Strain and Pressure [https://digitalcommons.library.uab.edu/etd-collection/479/]
- 99. Comprehensive First-Principles Study of Structural, Electronic, Magnetic, Elastic, Ferro-piezoelectricity, Thermodynamic, and Thermoelectric Properties of Hexagonal GaMnO3 Perovskite for Multiferroic Applications - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S2352214325001509]
- 100. - First to investigate the structural, elastic... principle calculations [https://link.springer.com/article/10.1007/s12648-023-02785-x]
- 101. First-principles calculations to investigate thermoelectric, thermophysical, and optical properties of RNi₄P₁₂ (R = Sm, Eu) rare-earth metal skutterudites | Scientific Reports [https://www.nature.com/articles/s41598-024-76538-z]
- 102. Machine learning for improved density ... functional theory [https://www.nature.com/articles/s41598-025-02088-7?error=cookies_not_supported&code=6c0634dd-ca66-4478-a996-2bdd0ff3b01a]
- 103. [2510.18754] Interplay of Magnetism, Band Gap Tuning, Optical, and Thermoelectric Responses in Fe-Doped YMnO$_3$: Insights from First-Principles Calculations [https://arxiv.org/abs/2510.18754]
- 104. Emerging New Drug Modalities in 2025 | BCG [https://www.bcg.com/publications/2025/emerging-new-drug-modalities]
- 105. FDA Drug Approvals : Full List of 28 New 2025 - GreyB Therapies [https://www.greyb.com/blog/fda-drug-approvals-2025/]
- 106. Novel Drug Approvals for 2025 | FDA [https://www.fda.gov/drugs/novel-drug-approvals-fda/novel-drug-approvals-2025]
- 107. Material Selection Chart - an overview | ScienceDirect Topics [https://www.sciencedirect.com/topics/materials-science/material-selection-chart]
- 108. Using semantics for representing experimental protocols | Journal of Biomedical Semantics | Full Text [https://jbiomedsem.biomedcentral.com/articles/10.1186/s13326-017-0160-y]